Introduction
The transfemoral approach is frequently used in endovascular therapies (EVT) such as percutaneous coronary intervention (PCI) and percutaneous peripheral intervention. Puncture site complications following transfemoral EVT include bleeding, haematoma, vessel occlusion and pseudoaneurysm formation, and remain a significant cause of patient morbidity in the perioperative period12. The traditional method of achieving haemostasis following EVT has been manual compression. Vascular closure devices have become available recently34. One of those devices, the Angio-Seal (Terumo) was introduced as a vascular closure device in Europe in 1994. It achieves haemostasis by compressing the puncture site between an anchor and a collagen plug56. In general, Angio-Seal use has been via retrograde puncture of the common femoral artery (CFA). However, antegrade punctures in transfemoral EVT are now frequently performed, especially in EVT for peripheral artery disease (PAD). The femoral puncture site in an antegrade puncture tends to be more proximal than in a retrograde puncture, such as in EVT for proximal superficial femoral artery (SFA) lesions. Hence, the risk of bleeding complications can be high because manual compression is difficult. Regardless, haemostasis is achieved by manual compression in most cases. The prevention of puncture site complications remains an important issue, and a more reliable haemostatic method is needed for daily clinical practice. In this paper, we discuss the modified interventional method of using a common antegrade femoral artery access for closure using an Angio-Seal device.
Methods
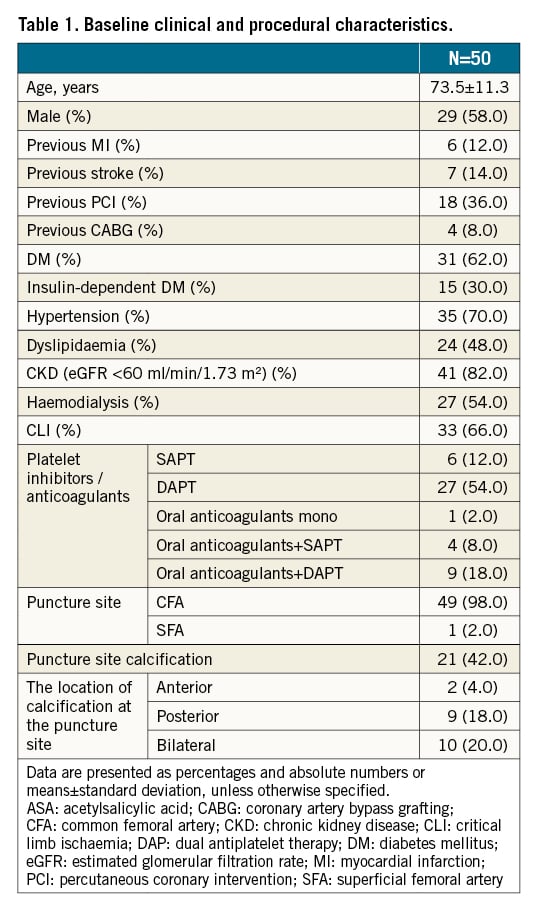
Between September 2017 and March 2018, we retrospectively analysed consecutive patients who underwent EVT with an antegrade puncture to the common femoral artery (CFA) using a 6 Fr introducer sheath. Patients with significant disease of the CFA were excluded at the operator’s discretion but 50 patients were included in this study. All patients suffered either from claudication or critical lower limb ischaemia due to PAD. Some of the patients were heparinised with 1,000-3,000 units intravenously. After EVT, all patients received a 6 Fr Angio-Seal vascular closure device. All procedures were performed with our new technique, described below. Patients remained in place for the following 3 hours. Clinical data were collected during hospital visits or by telephone contact at 6-month intervals. The relevant review boards in each institute approved the study protocol. We obtained written informed consent from each patient for data collection and analysis, in accordance with the Declaration of Helsinki.
The primary endpoint in this study was major adverse arterial events, defined as a composite of major bleeding, vessel occlusion and pseudoaneurysm formation. Major bleeding was defined as the occurrence of Bleeding Academic Research Consortium type 3 or 5 bleeding7. The patients were monitored during the follow-up period. Evaluation was focused on local findings including paresthaesia, pain, subcutaneous haematoma and pulse status of the femoral, popliteal, dorsal and posterior tibial arteries of the puncture site 24 hours, 6 weeks and 3 months after EVT.
The Modified AngiO-seal Haemostasis (MAOH) technique
The entire procedural detail of our novel method is basically the same as the standard technique. The major difference is that angiographic guidance is used to place the Angio-Seal outer sheath in the best position for the appropriate placement of the anchor (Figure 1A).
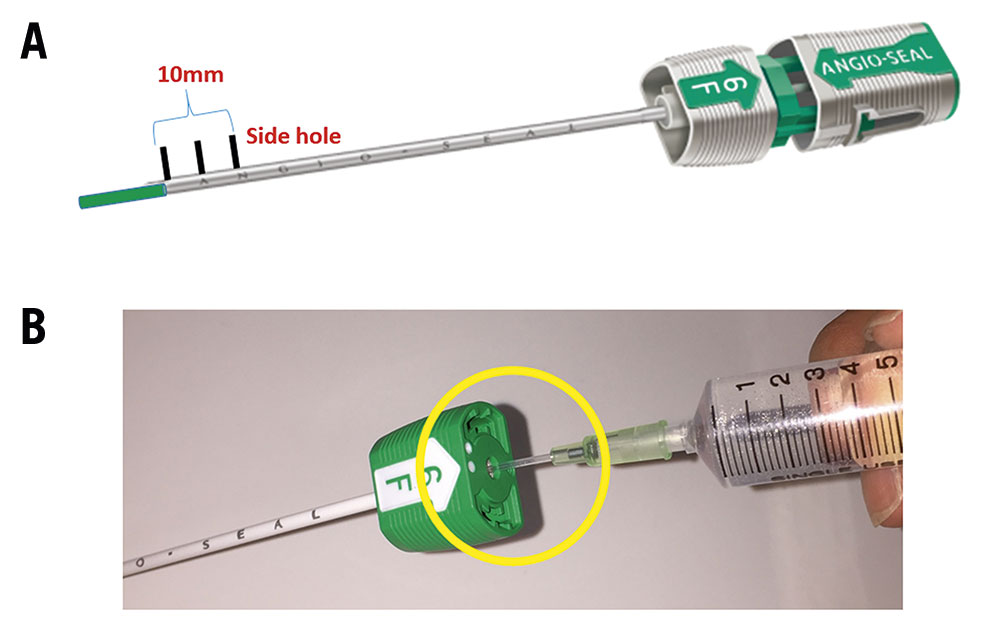
Figure 1. Representative images of the Angio-Seal device. A. Angio-Seal has three side holes at the tip of the outer sheath. The length from proximal to distal side hole is about 10 mm. B. Representative model of Angio-Seal outer sheath with inserted 18-gauge needle sheath
First, we inserted the Angio-Seal guidewire through the EVT sheath to the CFA. Second, we removed the EVT sheath, inserted the Angio-Seal outer sheath and removed the dilator and guidewire. Subsequently, as Figure 1B shows, we inserted an 18-gauge needle sheath into the Angio-Seal outer sheath and performed angiography (Moving image 1). Where this differs from the standard procedure is that we perform the angiography at an anterior oblique angle of about 60 degrees ipsilateral to the puncture site on a 6-inch screen. Under angiographic guidance, as Moving image 1 shows, we pulled back the Angio-Seal outer sheath until the extravasation of contrast from the side hole was observed. Finally, we inserted the anchor and the collagen plug, according to standard procedure. Figure 2 shows an idealised model of before (A) and after (B) the Angio-Seal outer sheath is pulled back.
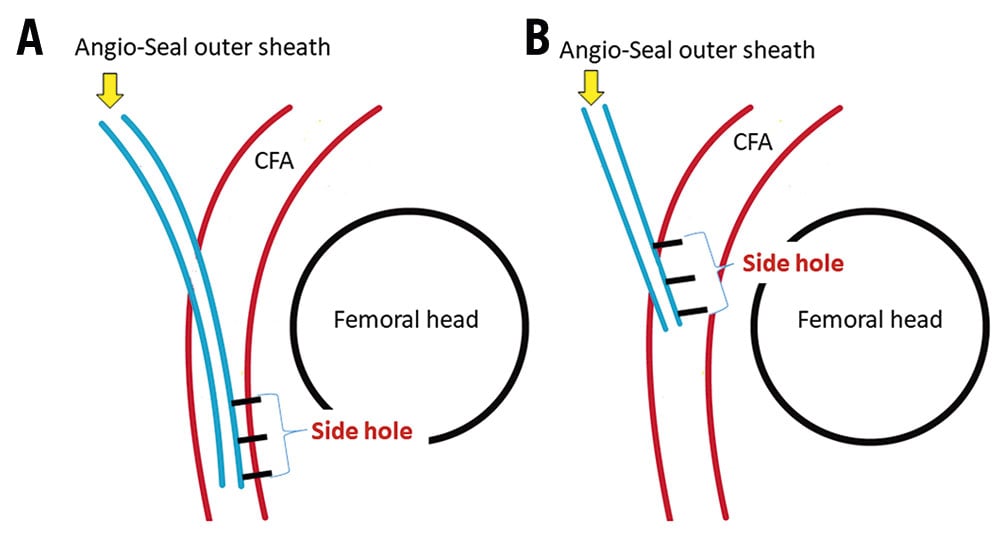
Figure 2. Ideal model of before and after the Angio-Seal device is pulled back. A. Outer sheath position before angiography with the Angio-Seal. B. The ideal Angio-Seal outer sheath position before the insertion of Angio-Seal anchor for appropriate placement of the anchor. Blue lines indicate the Angio-Seal outer sheath. Red lines indicate the common femoral artery (CFA). Three black lines indicate side holes of Angio-Seal outer sheath. The black circle indicates the femoral head. Both images are at an anterior oblique angle of 60° ipsilateral to the puncture site.
Results
Clinical and angiographic baseline characteristics are summarised in Table 1. In all patients, the Angio-Seal device was successfully deployed, and haemostasis was achieved within several minutes. The pouch enclosed the collagen plug, the collagen knot was palpable in all patients and no major complications occurred. Within the 24 hours following intervention, minor complications included transient oozing bleeding and mild pain. There was local groin pain around the puncture site in 4 of the 50 patients (8%) and a small subcutaneous haematoma in 3 patients (6%). At 3 months, no complications remained. A pulse deficit in the femoral, popliteal, dorsal and posterior tibial arteries of the puncture site was not observed in any of the patients at any time. Additionally, 4 patients had the CFA puncture within 90 days following index EVT with no complications occurring in these patients.
Discussion
We suggest that this modified interventional method using the Angio-Seal device increases the feasibility and efficacy of common antegrade femoral artery access closure.
We named the modified interventional haemostatic procedure the Modified AngiO-seal Haemostasis (MAOH) technique. Although previous studies reported similar findings8, our study is novel in that we introduce a modified technique that improves the success rate of the haemostatic procedure.
Initially, we tried to achieve haemostasis using the Angio-Seal device according to standard practice for antegrade CFA puncture, but the procedural success rate was very low. As illustrated in Figure 3, the following reasons were considered to be the causes of these procedural failures: first is the inappropriate placement of the anchor (Figure 3A). In cases where stents were placed in proximal SFA lesions, the collagen sponge was trapped at the calcified site of the artery due to a deep insertion of the Angio-Seal outer sheath. The second reason is the insufficient extrusion of the anchor (Figure 3B). This failure is due to an incomplete insertion of the Angio-Seal outer sheath into the artery. Both failures are due to the inappropriate positioning of the Angio-Seal outer sheath prior to the insertion of the Angio-Seal anchor. We suggest that the Angio-Seal outer sheath should be pulled back under angiographical guidance until the extravasation of the contrast dye from the side hole of the sheath is observed. The extravasation of the contrast dye indicates that the outer sheath is inserted 10 mm into the CFA. We consider that this is the best position for the outer sheath prior to the insertion and placement of the Angio-Seal anchor.
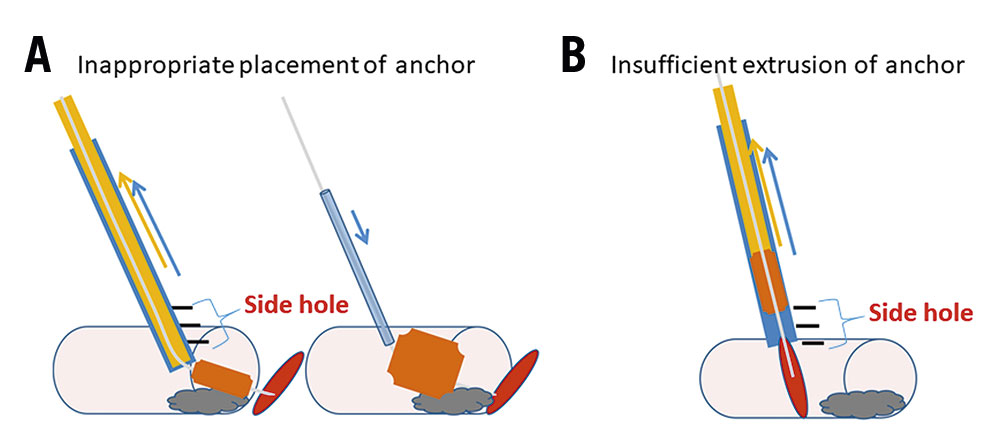
Figure 3. Representative image of Angio-Seal device failure. A. Inappropriate placement of anchor. A collagen sponge is trapped at the calcified site of the artery. B. Insufficient extrusion of anchor. The Angio-Seal outer sheath is incompletely inserted.
Achieving haemostasis with the Angio-Seal device has some advantages. Firstly, if haemostasis can be accomplished with the Angio-Seal device, the patient does not need to rest for a long time, and they do not feel discomfort or pain due to compression, which is different from the haemostasis by manual compression. Secondly, manual compression can lead to acute occlusion of the target vessel, but Angio-Seal avoids this risk. Thirdly, it might be especially useful in cases of critical limb ischaemia because lower extremity ischaemia due to compression can be avoided. Finally, in the case of proximal CFA puncture above the inguinal ligament, haemostasis with manual compression is often difficult, but it is not a problem if haemostasis is performed with the Angio-Seal.
In summary, we performed angiography using our MAOH technique, as in Moving image 1. The MAOH technique differentiates itself from the standard technique by allowing the outer sheath to be placed in the optimal position. In the standard method of angiography, the position of the outer sheath is often too deep or too shallow. The outer sheath position is very important for successful haemostasis with the Angio-Seal because inappropriate positioning of the outer sheath can lead to the failure of haemostasis. Finally, even with this novel technique of using the Angio-Seal device, access site outcomes may not be superior to manual compression alone because there is no comparison group using manual compression in this study. We want to emphasise some advantages mentioned above.
This study is limited by the small number of patients included and by the fact that it is a retrospective study; therefore, selection bias cannot be ruled out. A larger prospective database is needed to confirm these findings and compare effectiveness and safety among different closure devices.
Conclusions
We suggest that using the MAOH technique with the Angio-Seal device increases the feasibility and efficacy of the modified interventional method of antegrade CFA access closure.
Conflict of interest statement
The authors have no conflicts of interest to declare.
