Transcatheter valve-in-valve (ViV) procedures have become an effective option for failed surgical bioprosthesis implantation in order to avoid a second surgical valve replacement1. As ViV treatment becomes more common because of degenerative surgical valves, patients and doctors are increasingly choosing tissue valves as the first valve in aortic valve replacement. The INSPIRIS RESILIA aortic valve (Edwards Lifesciences) is the latest tissue valve with a novel technology of a valve frame that can expand to accomodate future ViV procedures. However, no published data on the performance of this expansion technology in ViV treatment are available. Here, we report a case of ViV treatment for a failed INSPIRIS RESILIA valve with insights from the postprcedural computed tomographic findings.
An 81-year-old female was admitted to undergo transcatheter valve-in-valve treatment for a calcified INSPIRIS RESILIA 23 mm valve, resulting in severe aortic stenosis with a peak velocity of 4.26 m/s and a mean pressure gradient of 43 mmHg. She had undergone surgical aortic valve replacement using a Perceval (LivaNova) valve, size M, 2 years earlier. She subsequently had another surgical aortic valve replacement due to infective endocarditis, and a 23 mm INSPIRIS RESILIA valve was implanted, concomitant with a Bentall procedure, 1 year and 4 months after the SAVR with the Perceval valve. Preprocedural computed tomography (CT) demonstrated that the area of the 23 mm INSPIRIS RESILIA was 343 mm2 (perimeter 65.7 mm) (Figure 1A) and showed leaflet calcification of the bioprosthesis (Figure 1B). Though virtual, the valve to coronary ostium distance was relatively short in both the right and left coronary arteries. We planned to implant a 26 mm SAPIEN 3 (Edwards Lifesciences) without coronary protection because she had a relatively enlarged sinus of Valsalva due to the Bentall procedure (Figure 1C). The 26 mm SAPIEN 3 was successfully implanted without any complications (Moving image 1, Moving image 2). Her dyspnoea disappeared, and she was discharged 7 days after the transcatheter aortic valve replacement (TAVR). Postprocedural echocardiography demonstrated a mean pressure gradient of 9 mmHg, and the index effective orifice area was 1.11 cm2/m2. The postprocedural CT demonstrated a funnel-shaped expansion throughout the transcatheter aortic valve without the expected frame expansion of the INSPIRIS (Figure 1D).
We demonstrated a ViV procedure for early structural valve deterioration of the INSPIRIS, which might have been caused by inflammation due to repeated surgical replacements, including the Bentall procedure. The INSPIRIS RESILIA aortic valve has an expansion zone that is designed to open to allow for upscaling in case of future ViV procedures, though no clinical data on frame expansion in ViV procedures are currently available2. In this case, however, the expansion zone of the INSPIRIS RESILIA did not expand, even after the implantation of a balloon-expandable valve. This might have happened because of an excessive amount of abnormal scar tissue caused by repeated surgical procedures, resulting in a rigid aortic annulus. Indeed, in the second aortic valve replacement for infective endocarditis, a bovine pericardial patch was used for the reconstruction of an abscessed aortic root, which might have interfered with the expansion. This is the first report of an unexpanded INSPIRIS frame during transcatheter valve-in-valve in a patient who underwent reconstruction of the aortic root.
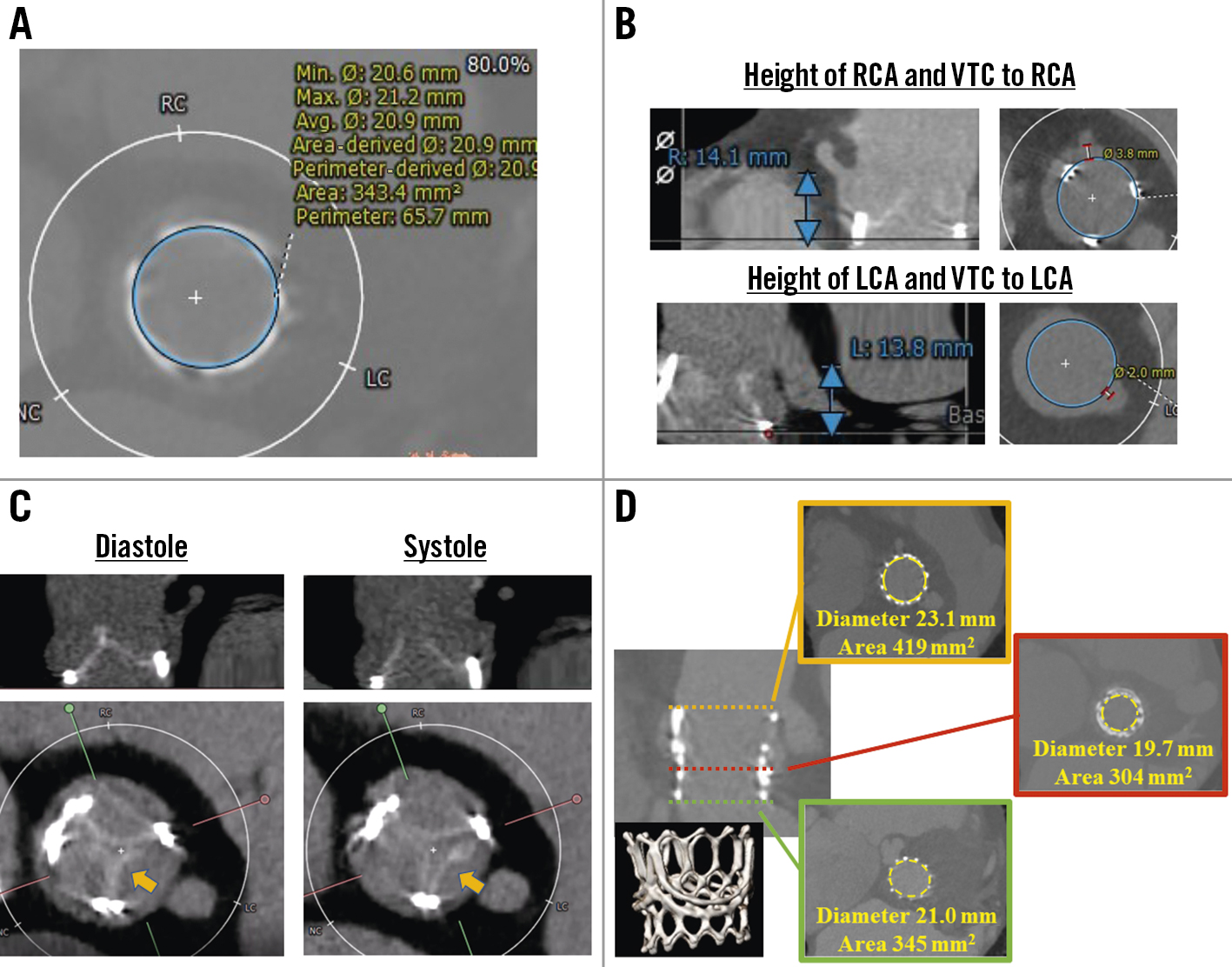
Figure 1. Computed tomographic images before and after transcatheter valve-in-valve procedure. A) Area and perimeter of the INSPIRIS RESILIA 23 mm valve. B) Coronary height and virtual transcatheter heart valve to coronary artery distance. C) Leaflet calcification on INSPIRIS RESILIA valve. The only leaflet located in native left coronary cusp opened during systolic phase (arrow). D) Postprocedural computed tomographic image demonstrating a funnel-shaped appearance of the SAPIEN 3 26 mm without frame expansion of the INSPIRIS RESILIA valve. LCA: left coronary artery; RCA: right coronary artery; VTC: valve-to-coronary distance
Conflict of interest statement
Y. Watanabe is aclinical proctor of transfemoral TAV for Edwards Lifesciences and Medtronic. The other authors have no conflicts of interest to declare.
