Introduction
The endovascular approach is becoming more common – and is associated with a lower periprocedural risk – in managing patients with peripheral arterial disease (PAD)1. Careful planning is crucial to the outcomes of endovascular procedures; several steps must be performed, including preplanning with vascular imaging using computed tomography angiography (CTA) or magnetic resonance angiography (MRA), choosing the right access and technique, and using the right devices (guidewire, sheath, catheter). Nowadays, more device options are available for vascular interventionists or vascular surgeons to choose from when performing peripheral interventions. On the other hand, in some environments with limited resources, vascular interventionists or vascular surgeons need to optimise the use of the available devices. Four important factors that may affect the choice of catheter and wire are laid out in the Central illustration. This review discusses how to choose the appropriate catheter and wire for a peripheral intervention.
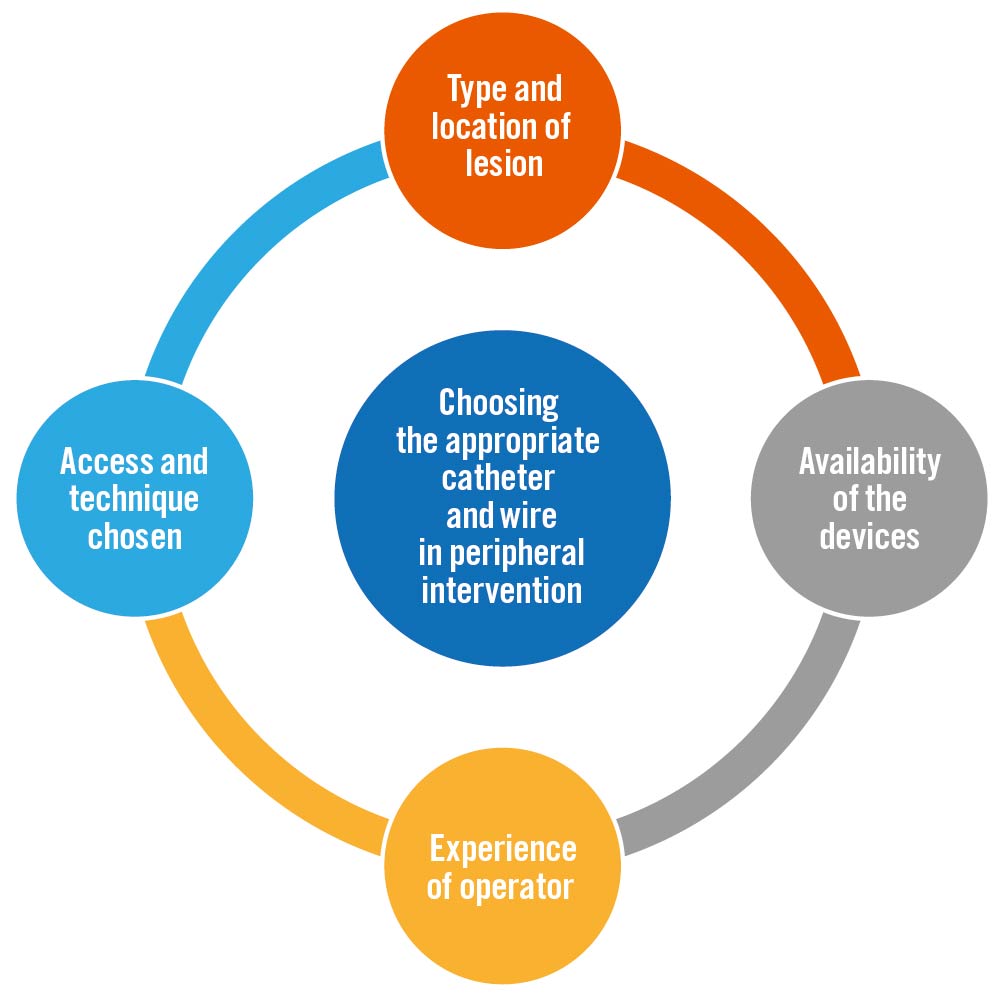
Central illustration. Choosing the appropriate catheter and wire in peripheral intervention. Important factors that need to be considered in choosing the appropriate catheter and wire for peripheral intervention.
Imaging for preplanning
CTA and MRA are non-invasive imaging modalities that are crucial in the preplanning of endovascular procedures for determining the length, characteristics and anatomical variations ok the lesion, as well as for identifying calcification2. Information from the non-invasive imaging guides us in the next two steps: (1) choosing the right access and technique and (2) choosing the appropriate devices. Both CTA and MRA have their own strengths and weaknesses. First of all, CTA and MRA with contrast technique have similarly high accuracies in classifying aortoiliac (both 92%) and femoropopliteal segments (94% and 90%, respectively), but CTA is preferred in patients with suspected lesions in the infrapopliteal segment because of its higher accuracy (92% vs 85%)3. The preferred imaging modality for endovascular procedure preplanning in below-the-knee (BTK) lesions, CTA is also preferred in mesenteric artery disease (acute and chronic) and extracranial carotid artery disease4. Although the study that compared the accuracy of CTA and MRA in diagnosing mesenteric artery disease (acute and chronic) has not yet been published, the accuracy of anatomical mapping with CTA was shown to be excellent, with 94% sensitivity and 95% specificity5. In addition, MRA is not always available outside office hours4. In extracranial carotid disease, CTA is superior to MRA and duplex ultrasonography because of its higher sensitivity and specificity in detecting carotid stenosis6.
CTA is also superior to MRA in several other aspects, including its wide availability, relatively shorter procedure duration, higher resolution, and the ability to be three-dimensionally (3D) reformatted. The downside of CTA is the radiation exposure and the use of iodinated contrast which might be harmful to chronic kidney disease (CKD) patients4. Instead, MRA with a non-contrast technique is useful in patients with kidney impairment (mild to moderate CKD) because it does not require the use of iodinated contrast. The downside of the MRA procedure is that more motion artefacts can be found due to the longer procedure duration. This procedure is contraindicated in patients with pacemakers and implantable cardioverter defibrillators47. During the procedure, digital substraction angiography can be helpful in preplanning the procedure for BTK lesion identification, particularly in patients with chronic limb-threatening ischaemia, due to the limitations of all other imaging to identify ankle or pedal segments appropriate for distal bypass4.
Angiosomes are 3D vessel networks found in all tissue layers including the skin, bone, and the layers between them8. In the BTK region, there are six angiosomes that are supplied by the branches of the three main arteries. The dorsum of the foot is supplied by the dorsalis pedis artery, as the continuation of anterior tibial artery. The foot instep is supplied by the medial plantar artery, the lateral midfoot and forefoot are supplied by the lateral plantar artery, and the heel is supplied by the calcaneal branch. The three arteries (medial plantar artery, lateral plantar artery, and calcaneal branch) are the continuation of the posterior tibial artery, while the lateral anterior upper ankle is supplied by one of the two branches of the peroneal artery. Another branch of the peroneal artery also supplies the heel (along with the posterior tibial artery’s calcaneal branch). Between the arteries there are collateral arteries which provide blood flow if the proximal artery is damaged2. If recognised, in certain conditions the angiosome can help the vascular interventionist or vascular surgeon to increase the procedural success. Direct angiosome revascularisations have been proven to positively affect vascular interventions, especially in critical limb ischaemia patients with below-the-knee lesions, by reducing the amputation rates and major adverse limb events (MALE), increasing the wound healing rates, and increasing the amputation-free survival (AFS)9. Hendawy et al reported that after 1-year follow-up of direct revascularisation (DR) and indirect revascularisation (IR), AFS and freedom from MALE were higher in the direct revascularisation group (75% vs 67% and 65% vs 55%, respectively)10. A systematic review including 10 studies comparing DR and IR conducted by Sumpio et al reported that DR was superior with regard to limb salvage and wound healing rates. However, direct angiosome revascularisation is not always necessary in patients with rest pain without tissue loss, tissue loss above the ankle, superficial ulceration (<10 mm in diameter), a fully intact pedal arch, and in diabetics, because indirect revascularisation is sufficient for treating these patients1112.
Duplex ultrasonography (DUS) is the least costly modality and does not require radiation exposure or contrast, unlike CTA and MRA. Several concerns, such as operator dependence and the method in which DUS data are presented, are hurdles to wider DUS adoption. A remarkable study conducted by de Vos et al found that PAD treatment planning based on CTA was mainly aligned with DUS-based treatment plans, while CTA was still thought to be essential to enhance confidence13. However, in the presence of calcified walls or plaques, duplex ultrasonography fails to classify stenosis14. Additionally, aortography also serves as a foundation for the ultimate judgment on whether intervention is required. Stenoses at the origins of the main branches of the aorta are the most common abnormalities that require aortography. Where there is a stenosis of more than 70%, an endovascular intervention is typically considered suitable15.
Basic peripheral intervention
Access and approach
The access route for the intervention is determined according to the location of the lesion and the experience of the operator. Radial access is the most common access for extracranial carotid artery disease and mesenteric artery disease1617. Radial access for vascular intervention is gaining interest due to its high safety record. Compared to femoral artery access, radial artery access was associated with a decreased incidence of bleeding problems, vascular access site challenges, and post-procedural blood transfusions. There are currently various haemostasis devices on the market, each effectively providing local pressure on the access site. The HemoBand (HemoBand), which is simply an adjustable plastic compression strap, is the most common. The RadiStop (Abbott) and Radstat (Wake Heart Associates) tourniquets are also used and are similar, but incorporate an adjustable screw for finer control of wrist pressure.
These haemostasis devices, however, must be withdrawn within two to three hours after placement. Because the radial artery has a smaller diameter and is more prone to tortuosity, smaller sheaths must be utilised during operations. Wires 0.018″ in diameter should always be advanced under fluoroscopic supervision since the radial artery’s branches are prone to perforation. Anatomical abnormalities such as recurrent radial and brachial loops, severe tortuosity, and auxiliary radial arteries may hinder wire and catheter passage in 5% of patients with these conditions18. Radial artery access has additional limitations, including restrictions on the length and location of endovascular devices. Long-shaft balloons and stents, and long introducer sheaths (up to 110 cm), should be available in catheterisation laboratories that conduct transradial peripheral interventions16.
On the other hand, in lower peripheral artery disease, femoral and popliteal access is more common, though radial access is also possible17. Access via the common femoral artery (CFA) is the most common procedure for endovascular treatment of the lower limb. One study suggested that if access to a patient’s CFA was found “hostile” due to obesity, extensive scar tissue, erythematous groin, high CFA bifurcation, and severe atherosclerosis or aneurysm in the CFA, access through the superficial femoral artery (SFA) should be pursued19. There are three common approaches used in lower peripheral intervention: antegrade, retrograde, and crossover/contralateral approach. These three approaches will be explained below.
The antegrade approach (Figure 1) is utilised for the ipsilateral non-ostial SFA, popliteal artery, and BTK vessels, but it is more commonly used and recommended for the latter2. The antegrade approach has more direct access to medial and distal BTK lesions. However, this approach is more technically challenging compared to other approaches because the distal flow is limited due to puncture site compression. Further, it is associated with a higher occurrence of small haematomas and bleeding2021; thus, in order to perform this approach, an experienced vascular interventionist/surgeon is needed. The approach begins with an antegrade puncture below the inguinal ligament. In several conditions, such as the presence of scar tissue, obesity, or a previously failed puncture, ultrasound can help decrease the procedure duration and increase the success rate22.
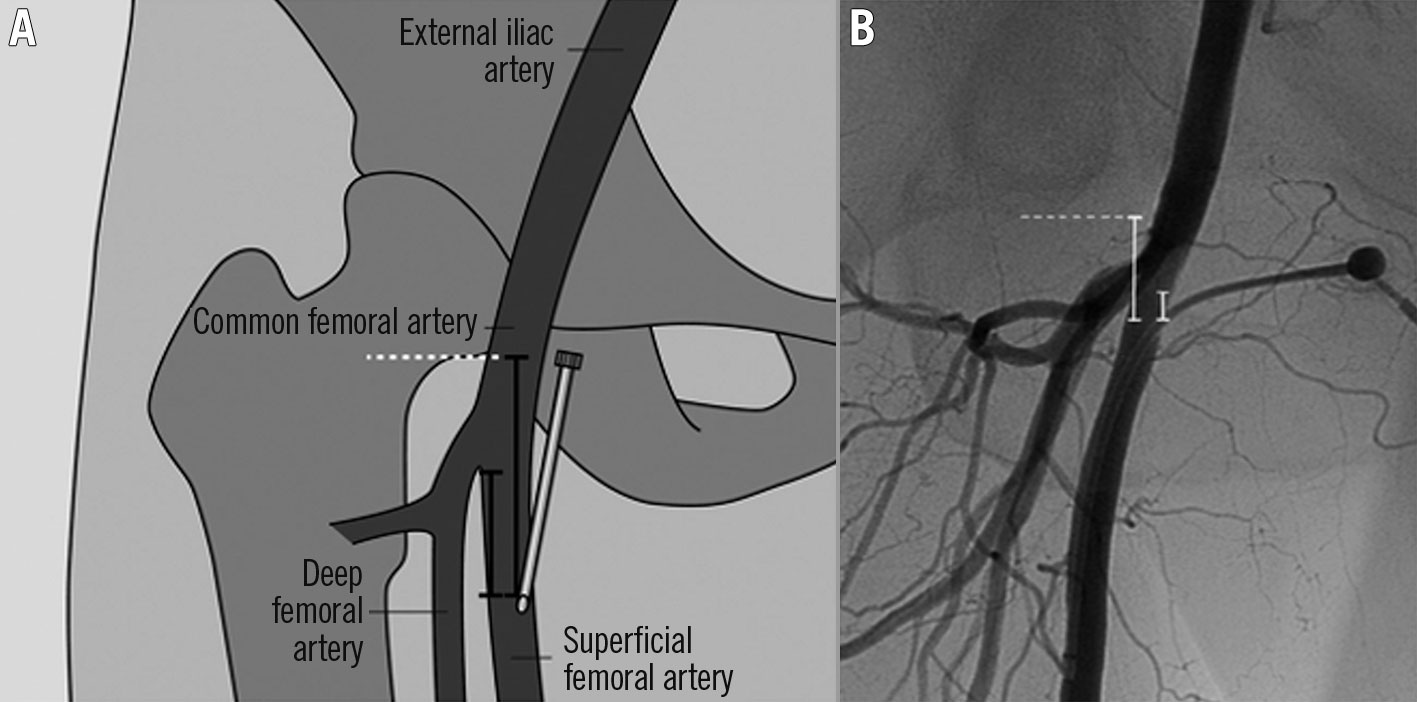
Figure 1. Illustration of the antegrade approach. A) Based on the angiographic image, the exact entry site into the SFA was determined in relation to the femoral arterial bifurcation and the lower margin of the femoral head (in mm). B) Corresponding angiographic image demonstrates the measurement between the arterial access and the lower margin of the femoral head and the femoral artery bifurcation, respectively (in mm). SFA: superficial femoral artery. Reproduced from Gutzeit et al19, with permission.
The retrograde approach (Figure 2) is the inverse approach of the antegrade approach and can be performed from the femoral, popliteal, and dorsalis pedis/tibial arteries. Thus, this approach is helpful for lesions located in the distal aorta, ipsilateral common iliac, proximal-to-mid external iliac, ipsilateral vessels proximal to the distal branch of the SFA, and tibial vessels. This approach can be performed as another attempt after failing to cross a chronic total occlusion (CTO) lesion with the antegrade approach2. A study conducted by Matsumi et al found that the long-term outcomes of chronic limb ischaemia (CLI) patients with BTK-CTO lesions undergoing an endovascular procedure via the retrograde approach after a failed attempt with antegrade approach were promising, with an AFS rate of 78.6% at 1 year and 50.2% at 5 years after the procedure23. During the approach, the foot needs to be stabilised with tape. A micropuncture access setup is generally prepared and a nitroglycerine injection is performed via the proximal access catheter. Ultrasound is important during this approach, especially when performed through the dorsalis pedis24. Local anaesthesia is required with a 25 G (0.018″) micropuncture. In performing this procedure, sometimes a sheath is not necessary25. Ultrasound guidance is becoming more effective in guiding punctures for all kinds of vascular access, as shown by the growing body of research. A professional operator and an appropriate technique are required for a successful intervention and to prevent complications26.
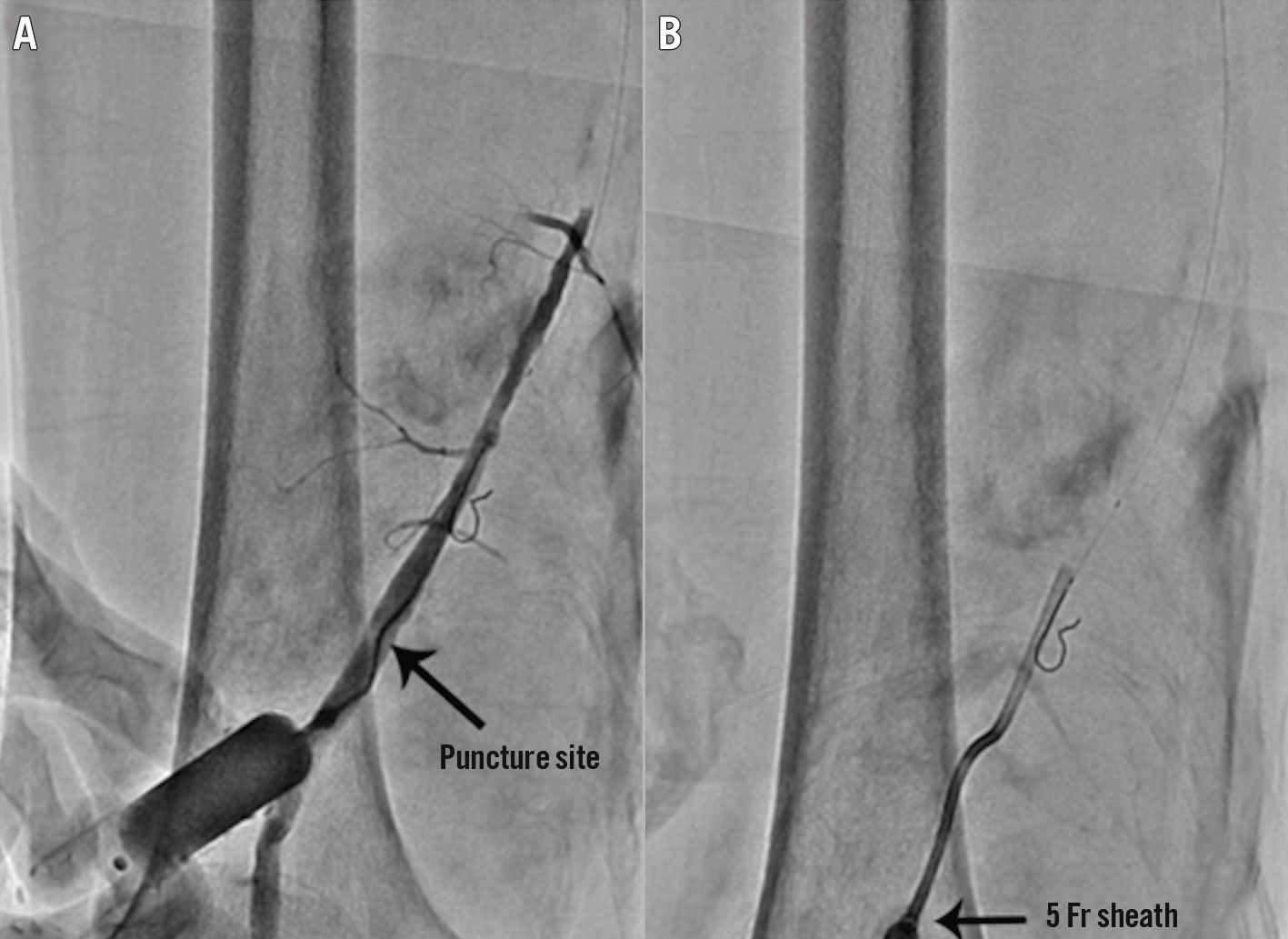
Figure 2. Illustration of the retrograde approach. The distal SFA is punctured at the proximal part of the adductor canal with a 21-gauge micropuncture needle under roadmap fluoroscopic guidance, and an angiogram is performed through a 3 Fr dilator to confirm that the access to the SFA is achieved properly (A). A 5 Fr sheath is then inserted into the distal SFA (B). SFA: superficial femoral artery. Reproduced from Shin et al40, with permission.
The crossover/contralateral approach (Figure 3) is an easier approach and is associated with less challenging treatment for bleeding compared to antegrade approaches2728. The target vessels for this approach are the contralateral internal iliac, distal common iliac, external iliac, femoral, popliteal, and below-the-knee vessels. This approach has better access to proximal lesions. Furthermore, this approach keeps the puncture site apart from the treated segment and allows the use of closure devices in a more secure way27. Conversely, multicentre research revealed that antegrade access is significantly linked to a much-reduced risk of access site complications compared to the crossover technique. There are many reasons why access complications are more significant in the crossover group, including the difficulty of inserting 90 cm-long sheaths via contralateral access, notably in iliac arteries that are elongated or calcified28. According to other research, 6.6% of patients treated using an antegrade approach had an access site haematoma, compared to 7.1% of patients treated using a crossover approach29.
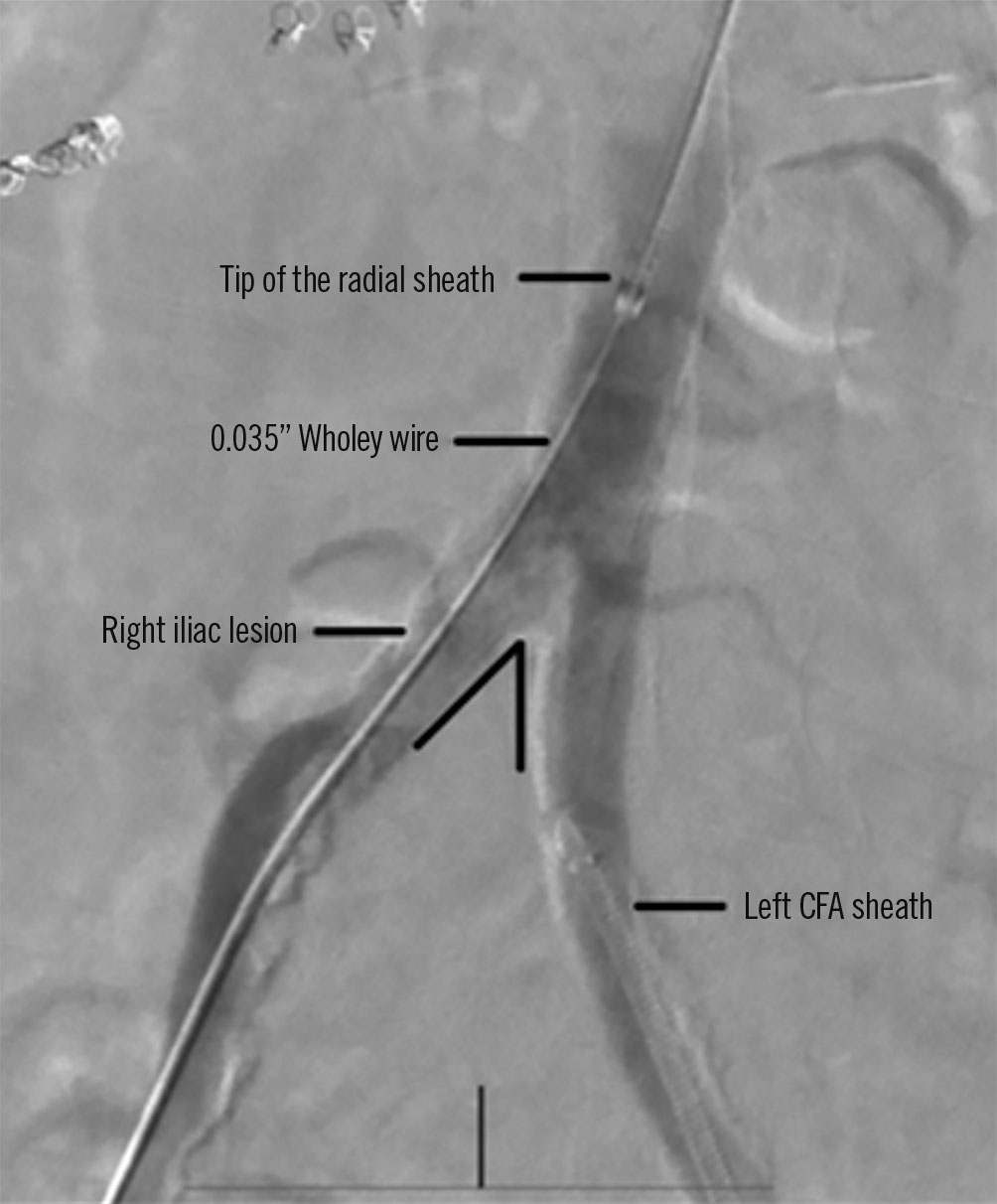
Figure 3. Illustration of the crossover/contralateral approach. Radial access for acute angulation of the abdominal aortic bifurcation. Note the acute angulation of the abdominal aortic bifurcation. CFA: common femoral artery. Reproduced from Hammad et al41, with permission.
In addition, the insertion of a sheath preserves a constant source of arterial access. Sheath sizes range from 4 Fr to 24 Fr according to the puncture site. Catheter size is often determined by operator preference and patient anatomy. Some operators choose to use 4 Fr systems for all diagnostic procedures, barring any patient pathology that may require a larger diameter system. Generally, the sheath size is kept as small as possible to minimise the vascular puncture and reduce complications30.
Most occlusion balloons accept the 4 Fr sheath in interventions for BTK arteries. Femoral access, on the other hand, provides additional flexibility in the event of a complicated intervention or emergency such as complex bifurcation lesions or rotational atherectomy. A sheath and catheter with a 7 or 8 Fr diameter may be necessary for these situations. In obese individuals, radial access is an alternative to the femoral approach. The sheath size is limited by the radial artery lumen, which is on the small side at 2 mm. Therefore, it is recommended that a maximum of 6 Fr for the sheath should not be exceeded when using radial access31.
Intraluminal vs subintimal techniques
Intraluminal and subintimal techniques are the two most common techniques in addressing CTO. Both have their own pros and cons, which were thoroughly explained in a review conducted by J.P. Pigott. Occluded lumen composition and structures influence the choice of wire. Intraluminal wires can usually pass through soft plaque or intraluminal thrombi, but harder plaques like fibrotic or calcific plaque make the intraluminal techniques more challenging. The procedural cost is minimal with the intraluminal technique, with a crossing catheter and standard guidewire needed to complete the procedure. One concern with the intraluminal technique is that it is not always effective and switching to a subintimal technique may be needed to achieve a successful outcome. It is also impossible to perform CTO crossing without using concurrent imaging to ensure that the guidewire does not deviate into subintimal channels. The intraluminal technique often requires additional therapy other than an angioplasty balloon. The plaque may need to be debulked or modified using atherectomy devices32.
Therefore, in long CTO lesions, the subintimal technique should be prioritised because crossing with the intraluminal technique is nearly impossible24. The subintimal technique was first applied in femoropopliteal lesions but it can also be applied in other locations including the iliac and mesenteric arteries2633. Additional advantages of the subintimal technique include a shorter operation duration without increased perioperative problems or a loss in primary patency. The subintimal technique has improved as a result of improvements in several methods and devices, resulting in higher success and patency rates for femoropopliteal CTO lesions in terms of technical success34.
Soga et al discovered no statistically significant difference in 3-year primary patency between the intraluminal (55%) and subintimal (53%) groups. The 3-year assisted primary patency rate in the two groups was also comparable (65% of the intraluminal and 74% of the subintimal group). The secondary patency was similar after three years (80% of the intraluminal and 85% of the subintimal group). Overall survival after three years did not differ substantially between the two groups35.
A study was done to compare the results of drug-eluting stent (DES) implantation using subintimal vs intraluminal methods for femoropopliteal CTO. The rates of first- and second-year restenosis following subintimal or intraluminal DES implantation were similar. However, this study suggests that regardless of the patient, lesion, or procedural factors, subintimal DES implantation seems to be therapeutically feasible36.
Devices in peripheral intervention
Guidewires
Guidewires function as guidance for catheter or sheath insertions to the vessel. This device is very useful in stenoses and occlusions. The wire consists of a metal core and an outer wire wrapped around its metal core. The stiffness of the guidewire is determined by its metal core15. The two most common guidewires used today are the 0.035″ and 0.014″ wire. The 0.035″ wire is usually used for subclavian, innominate, iliac, superficial femoral, and popliteal artery interventions. While the 0.014″ wire is generally used for carotid, vertebral, renal, and below-the-knee interventions. 0.018″ wires are used less often for renal, subclavian, and popliteal interventions37.
Guidewires serve as an important “rail”, particularly for balloon catheters. Balloon catheters are almost always made straight, and as such, can only be directed through curves with the help of a guidewire. In certain cases, the most suitable guidewire may have a bend at its tip and should be chosen for rotational stability, such as a thin steel wire or glidewire. The tips of thin 0.014″ or 0.018″ steel wires bend easily. However, the tip can easily distort when it comes into contact with resistance, making such a wire useless. To overcome this challenge, the Glidewire Advantage (Terumo) has a tip with a nitinol core, which is substantially more resistant to deformation15.
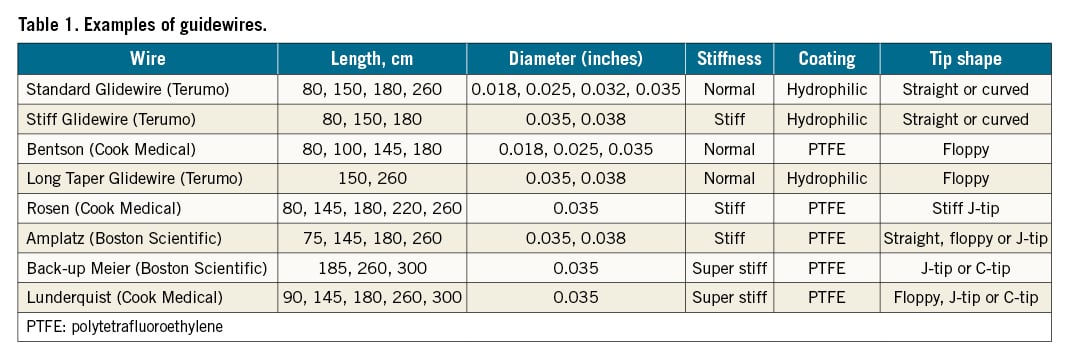
The Glidewire is unique compared to previous guidewires because of its different structure. The Glidewire has a nitinol (nickel/titanium alloy) core with plastic sheathing that has a hydrophilic outer coating wrapped around it. This structure offers several advantages, including being largely resistant to kinking, being more reliable when guiding a catheter or sheath through difficult curves or across a bifurcation, and its hydrophilic coating allows it to be used in the context of high-grade stenosis or occluded vessels15. Moreover, compared to other hydrophilic guidewires, the Glidewire has the lowest potential for perforation38. The disadvantage of the Glidewire is that its surface is made of plastic and thus is more vulnerable compared to other guidewires whose exteriors are made of wire, and must be used with caution when combined with sharp cannula. Although the Glidewire is largely resistant to kinking, if the wire is forcefully kinked, a slight bend will remain in the Glidewire. This slight bend can be helpful in certain conditions such as when guiding a balloon catheter past the deep femoral artery to the superficial femoral artery in crossover catheterisation and approaching the right subclavian artery from the brachiocephalic trunk15. A stiff guidewire (e.g., Lunderquist [Cook Medical] or Amplatz [Boston Scientific]) on the other hand, is advantageous for a tortuous vascular anatomy since it is highly dirigible, trackable, and gives enough support37. A selection of guidewires is outlined in Table 1.
Sheath
A sheath is a catheter with a thin wall, a valve, and a lateral connection which slides through the guidewire after being introduced into the vessel using a dilator. It is used for vascular wall protection during interventions, but is not required in every intervention. A sheath can be utilised maximally in conditions where the intervention was planned to be executed with several catheter changes or when there is a very hard vascular wall. A sheath consists of a one-way haemostatic valve, a dilator, and a sidearm port1539. The haemostatic valve reduces the risk of air and atherosclerotic debris embolisation by allowing blood to bleed back when the valve opens37. The dilator’s role is to stiffen the sheath during placement, while the sidearm port’s role is to administer the medication or contrast 30. Sheaths are sized according to their inner diameter (differentiated from catheters which are sized according to their outer diameter)15. In order to ensure a successful operation, an interventional cardiologist must meticulously consider and decide on every aspect of the sheath, including its diameter. Individual patient vascular geometry needs an appropriate sheath size that minimises damage to the artery and minimises haemostasis issues, giving higher patient comfort and early ambulation30.
In lower peripheral interventions, guiding sheath sizes are the first thing that clearly differ between the ipsilateral/antegrade approach and the contralateral/crossover approach. The ipsilateral/antegrade approach needs a shorter sheath (30-55 cm) compared to the contralateral/crossover approach, which requires a longer sheath (70-90 cm). Different lesion locations call for different sizes of sheath to be used. For the antegrade approach, a 6 Fr guiding sheath is used for an SFA lesion and a 5 Fr for a BTK lesion. On the other hand, the contralateral approach needs a bigger guiding sheath. The 6/7 Fr Arrow (Teleflex), Fortress (Biotronik), Flexor Ansel (Cook Medical), and Heartrail (Terumo) are the most common guiding sheaths for the antegrade approach; the first three guiding sheaths on this list are also used in the contralateral approach. Destination (Terumo) is another guiding sheath that may be used in the contralateral approach.
Angiographic catheters
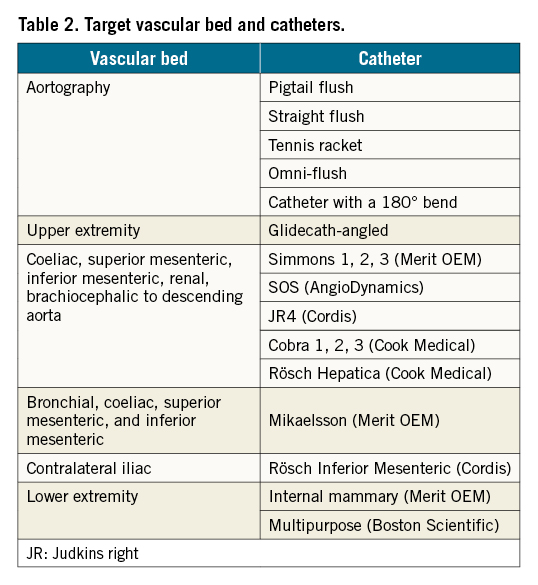
The angiography catheter is a crucial device in peripheral interventions because it transports contrast agents to vascular lesions in order to visualise the lesion prior to the intervention15. Angiographic catheters can be divided into three subtypes of catheters: flush, exchange, and selective catheters. Flush catheters are useful for high-pressure injections (up to 1,200 psi), especially in the aorta, which has a very high pressure. Straight catheters are used for three main objectives: as a general exchange tool, for interval arteriography, and to measure pressure in the distal and proximal areas of the lesion39. Selective catheters are used for smaller arteries. Advances in selective catheter design have improved their rotational stability, and the wide variety of catheter shapes allows for catheterisation in almost every artery in the body from superior to inferior extremity access 15. Catheters and their corresponding vascular beds are listed in Table 2.
Conclusions
A thorough preparation including performing CTA or MRA is crucial for choosing appropriate catheters and wires during a peripheral intervention. Angiosomes can be useful in predicting the site of the lesion prior to CTA or MRA in a lower peripheral intervention. Access and technique should be chosen wisely according to the type and location of the lesion, and the experience of the vascular interventionist or vascular surgeon. The choice of guidewire, sheath, and catheter will follow from the initial information gathered, the access and technique chosen, and the availability of the devices. The length, diameter, stiffness, coating, and the tip shape of the guidewire should be adjusted according to the initial information and preparation. A sheath is not always necessary but will be beneficial when several catheter changes are planned. There are three types of angiographic catheters (flush, exchange, and selective) which should be considered and used according to their function.
Acknowledgements
The authors thank all who supported this article review.
Conflict of interest statement
The authors have no conflicts of interest to declare.

