Results
Patient population
Between June 2015 and May 2018, 1,113 patients with true bifurcation lesions were screened (Figure 1). Of them, 825 patients were excluded: chronic total occlusions (CTOs) in 89 patients (60 CTOs in the MV, 16 in the SB, and 12 in both the MV and the SB); a two-stent approach in 9 patients; poor imaging quality in 192 patients; and 535 undergoing SB treatment (including predilation or kissing balloon inflation or stenting). Finally, 288 patients were included in this study. Immediately after the PCI procedures, 65 (22.6%) patients had an SB μQFR <0.8 and 223 (77.4%) patients had an SB μQFR ≥0.8.
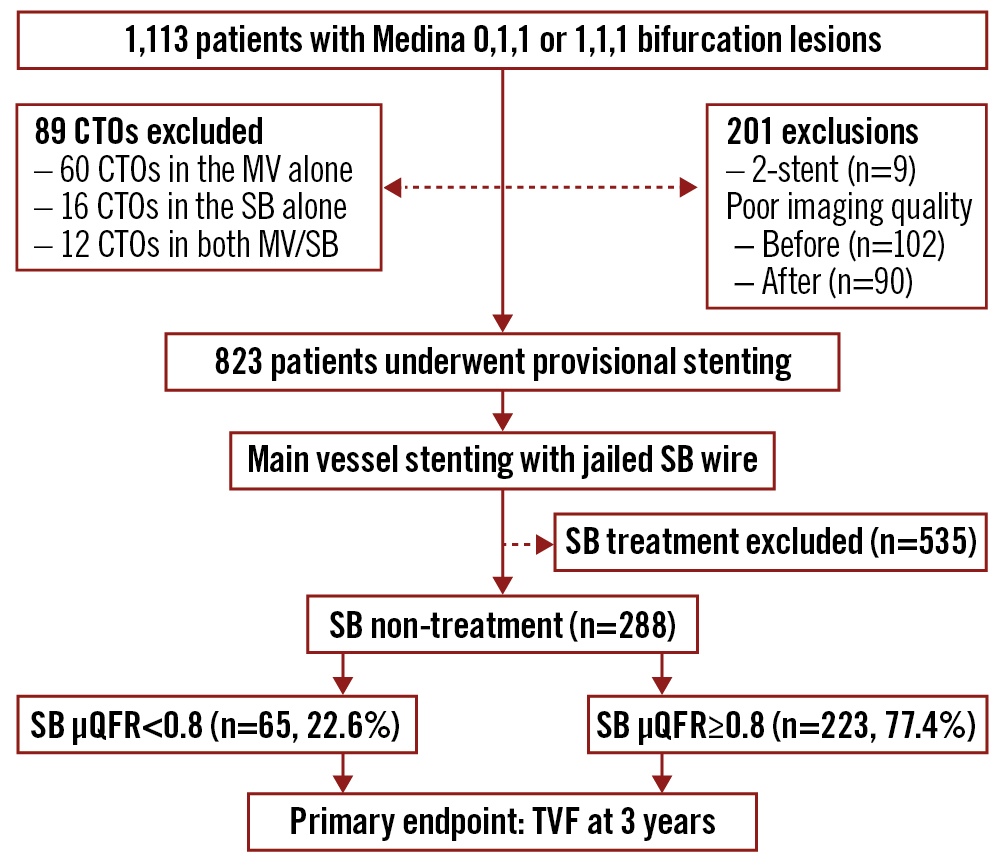
Figure 1. Study flowchart. Of 288 patients with true coronary bifurcation lesions after stenting the main vessel (MV) without side branch (SB) treatment (including predilation, or kissing balloon inflation or stenting), 65 patients had an SB quantitative flow ratio (μQFR) of <0.8 and 223 patients had an SB μQFR ≥0.8. CTO: chronic total occlusion; TVF: target vessel failure
Baseline clinical characteristics
Patients in the SB μQFR <0.8 group were older (66.4±10.0 years vs 64.4±9.9 years; p=0.017) and had more frequent previous PCI (32.3% vs 13.0%; p=0.001), compared to patients in the SB μQFR ≥0.8 group (Table 1).
Table 1. Baseline characteristics of patients.
| SB μQFR <0.8 (n=65) | SB μQFR ≥0.8 (n=223) | p-value | ||
|---|---|---|---|---|
| Age, years | 66.4±10.0 | 64.4±9.9 | 0.017 | |
| Male, n (%) | 51 (78.5) | 165 (74.0) | 0.518 | |
| Body mass index, kg/m2 | 24.6±3.1 | 24.6±2.9 | 0.629 | |
| Body surface area, m2 | 1.86±0.16 | 1.86±0.17 | 0.888 | |
| Hypertension, n (%) | 44 (67.7) | 144 (64.6) | 0.767 | |
| Diabetes, n (%) | 23 (35.4) | 69 (30.9) | 0.546 | |
| Hyperlipidaemia, n (%) | 34 (52.3) | 107 (48.0) | 0.575 | |
| Previous MI, n (%) | 10 (15.4) | 30 (13.5) | 0.686 | |
| Previous PCI, n (%) | 21 (32.3) | 29 (13.0) | 0.001 | |
| Previous CABG, n (%) | 0 | 2 (0.9) | 1.000 | |
| Renal dysfunction, n (%) | 4 (6.2) | 8 (3.6) | 0.478 | |
| Current smoker, n (%) | 14 (21.5) | 41 (18.4) | 0.592 | |
| Family history, n (%) | 2 (3.1) | 11 (4.9) | 0.739 | |
| GI bleeding, n (%) | 2 (3.1) | 5 (2.2) | 0.658 | |
| Stroke, n (%) | 7 (10.8) | 24 (10.8) | 1.000 | |
| Peripheral artery disease, n (%) | 1 (1.5) | 17 (7.6) | 0.085 | |
| Heart failure, n (%) | 8 (12.3) | 18 (12.6) | 1.000 | |
| LVEF, % | 59.4±8.4 | 60.8±7.5 | 0.583 | |
| Heart rate, bpm | 72.7±10.9 | 74.1±11.1 | 0.695 | |
| Systolic blood pressure, mmHg | 135.9±17.9 | 131.8±17.3 | 0.300 | |
| Diastolic blood pressure, mmHg | 77.9±10.2 | 78.6±10.5 | 0.909 | |
| Clinical presentation, n (%) | Silent ischaemia | 3 (4.6) | 11 (4.9) | 1.000 |
| Stable angina | 13 (20.0) | 42 (18.8) | 0.858 | |
| Unstable angina | 34 (52.3) | 122 (54.7) | 0.778 | |
| AMI >24 h | 15 (23.1) | 48 (21.5) | 0.865 | |
| STEMI | 6 (9.2) | 25 (11.2) | 0.821 | |
| NSTEMI | 9 (13.8) | 23 (10.3) | 0.500 | |
| Data are mean±standard deviation or n (%). AMI: acute myocardial infarction; CABG: coronary artery bypass graft; GI: gastrointestinal; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NSTEMI: non-ST-elevation myocardial infarction; PCI: percutaneous coronary intervention; μQFR: novel quantitative flow ratio; SB: side branch; STEMI: ST-segment elevation myocardial infarction | ||||
LESION and procedural characteristics
The lesion length in the MV was 37.5±12.4 mm in the SB μQFR <0.8 group (Table 2), significantly longer than the 34.9±17.4 mm in the SB μQFR ≥0.8 group (p=0.022). Baseline diameter stenosis at the ostial SB (59.9% vs 52.0%; p=0.072) and SB lesion length (14.5±7.1 vs 13.4±9.2; p=0.382) were comparable between the two groups. More lesions needed to be treated (2.23±0.86 vs 1.92±0.82; p<0.001) in the SB μQFR <0.8 group, resulting in a higher rate of staged procedures (40.0% vs 26.0%; p=0.043) and fewer complete revascularisations (40.0% vs 63.7%; p=0.001). Most procedures were performed using the transradial approach. Intravscular ultrasound guidance was used in fewer than 30% of patients.
Table 2. Lesions and procedural characteristics.
| SB μQFR <0.8 (n=65) | SB μQFR ≥0.8 (n=223) | p-value | ||
|---|---|---|---|---|
| No. of diseased vessels | Single-vessel disease, n (%) | 12 (18.5) | 61 (27.4) | 0.194 |
| Two-vessel disease, n (%) | 29 (44.6) | 103 (46.2) | 0.888 | |
| Three-vessel disease, n (%) | 24 (36.9) | 59 (26.5) | 0.120 | |
| LM bifurcation lesions, n (%) | 20 (30.8) | 79 (35.4) | 0.554 | |
| Moderate-severe calcification | Main vessel, n (%) | 20 (30.8) | 68 (30.5) | 1.000 |
| Side branch, n (%) | 9 (13.8) | 19 (8.5) | 0.234 | |
| Thrombus-containing lesion | Main vessel, n (%) | 2 (3.1) | 8 (3.6) | 1.000 |
| Side branch, n (%) | 1 (1.5) | 0 | 0.226 | |
| TIMI flow grade 3 prior to procedure | Main vessel, n (%) | 59 (90.8) | 200 (89.7) | 0.823 |
| Side branch, n (%) | 62 (95.4) | 213 (95.5) | 0.334 | |
| Lesion length | Main vessel, mm | 37.5±12.4 | 34.9±17.4 | 0.022 |
| Side branch, mm | 14.5±7.1 | 13.4±9.2 | 0.382 | |
| Diameter stenosis | Main vessel, % | 54.3±14.0 | 52.2±17.8 | 0.243 |
| Side branch, % | 59.9±13.9 | 52.0±19.2 | 0.072 | |
| No. of lesions, n | 2.34±0.87 | 2.14±0.91 | <0.001 | |
| No. of treated lesions, n | 2.23±0.86 | 1.92±0.82 | <0.001 | |
| Transradial access, n (%) | 50 (76.9) | 187 (83.9) | 0.201 | |
| IVUS guidance, n (%) | 18 (27.7) | 66 (29.6) | 0.877 | |
| IABP, n (%) | 0 | 1 (0.4) | 1.000 | |
| MV TIMI flow grade 3 post-procedure, n (%) | 64 (98.5) | 222 (99.6) | 0.862 | |
| SB TIMI flow grade 3 post-procedure, n (%) | 50 (76.9) | 220 (98.7) | <0.001 | |
| Staged PCI, n (%) | 26 (40.0) | 58 (26.0) | 0.043 | |
| Complete revascularisation, n (%) | 26 (40.0) | 142 (63.7) | 0.001 | |
| Data are mean±standard deviation or n (%). IABP: intra-aortic balloon pumping; IVUS: intravascular ultrasound; LM: left main; MV: main vessel; PCI: percutaneous coronary intervention; μQFR: novel quantitative flow ratio; SB: side branch; TIMI: Thrombolysis in Myocardial Infarction | ||||
Dynamic change of μQFR
The inter- and intraobserver variances were less than 5%.
At baseline, the absolute value of μQFR in the SB μQFR <0.8 group was lower than that in the SB μQFR ≥0.8 group (0.61±0.19 vs 0.71±0.22; p=0.001) (Table 3), and the percentage of patients with an SB μQFR <0.8 was 90.8% (n=59), significantly different from the 59.2% (n=132) in the SB μQFR ≥0.8 group (p<0.001). However, the percentage of patients with a baseline MV μQFR <0.8 did not differ significantly between the two groups.
After stenting the MV and POT, the μQFR in the MV increased to 0.93±0.07 in the SB μQFR ≥0.8 group, compared to 0.91±0.09 (p=0.008) in the SB μQFR <0.8 group, resulting in a higher rate of μQFR <0.89 in the SB μQFR <0.8 group (23.1% vs 12.6%; p=0.047).
For the SB, immediately after stenting the MV and POT, a more profound increase of μQFR in the SB was measured in the SB μQFR ≥0.8 group (0.20±0.22), compared to 0.03±0.21 in the SB μQFR <0.8 group (p<0.001). Baseline diameter stenosis at the ostial SB (odds ratio [OR] 9.55, 95% CI: 1.51-15.92; p=0.023) and SB lesion length (OR 5.433, 95% CI: 1.201-10.93; p<0.001) were the two predictors of a μQFR <0.8 in the SB immediately after stenting the MV.
Table 3. Dynamic change of quantitative flow ratio.
| SB μQFR <0.8 (n=65) | SB μQFR ≥0.8 (n=223) | p-value | ||
|---|---|---|---|---|
| Target vessel, n (%) | LAD-LCx | 16 (24.6) | 65 (29.1) | 0.386 |
| LAD-diagonal | 36 (55.4) | 128 (57.4) | ||
| LCx-obtuse marginal | 11 (16.9) | 21 (9.4) | ||
| Distal RCA | 2 (3.1) | 9 (4.0) | ||
| Main vessel μQFR | Baseline | 0.61±0.22 | 0.61±0.24 | 0.046 |
| <0.8, n (%) | 46 (70.8) | 167 (74.9) | 0.523 | |
| Post-procedure | 0.91±0.09 | 0.93±0.07 | 0.008 | |
| <0.89, n (%) | 15 (23.1) | 28 (12.6) | 0.047 | |
| Side branch μQFR | Baseline | 0.61±0.19 | 0.71±0.22 | 0.001 |
| <0.8, n (%) | 59 (90.8) | 132 (59.2) | <0.001 | |
| Post-procedure | 0.64±0.14 | 0.91±0.06 | <0.001 | |
| ∆μQFR | 0.03±0.21 | 0.20±0.22 | <0.001 | |
| Data are mean±standard deviation or n (%). LAD: left anterior descending artery; LCx: left circumflex; MV: main vessel; μQFR: novel quantitative flow ratio; RCA: right coronary artery; SB: side branch | ||||
Primary and secondary endpoints
At 30 days, the rate of endpoints was comparable between the two groups after adjusted analysis (Table 4). Within one-year after stenting, the incidence of TVMI and TVR in the SB μQFR <0.8 group were 15.4% and 12.3%, respectively, significantly different to the 4.9% and 1.3% in the SB μQFR ≥0.8 group, by either unadjusted or adjusted analyses.
At 3-year follow-up, TVF was reported in 43 (20.0%) patients overall, with 29.2% of patients in the SB μQFR <0.8 group and 10.8% in the SB μQFR ≥0.8 group (adjusted HR 2.45, 95% CI: 1.30-5.53; p=0.003) experiencing TVF, largely driven by increased rates of TVMI (16.9% vs 5.4%, adjusted HR 3.29, 95% CI: 1.15-6.09; p=0.030) and TVR (15.4% vs 2.2%, adjusted HR 6.39, 95% CI: 2.04-13.48; p=0.007) (Table 4, Figure 2). Landmark analysis between the two groups (Figure 3) showed a significant difference in TVF within 30 days and at one year but not between one and three years.
By multivariate analysis, previous PCI (OR 4.81, 95% CI: 1.07-21.69; p=0.041) and an SB μQFR <0.8 (OR 6.88, 95% CI: 2.09-22.64; p=0.002) were the two independent factors of 3-year TVF.
Table 4. Primary and secondary endpoints.
| SB μQFR <0.8 | SB μQFR ≥0.8 | Unadjusted analysis | Adjusted analysis | |||
|---|---|---|---|---|---|---|
| (n=65) | (n=223) | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| At 30 days | ||||||
| TVF | 9 (13.8) | 13 (5.8) | 2.59 (1.06-6.38) | 0.038 | 2.44 (0.67-5.25) | 0.162 |
| Cardiac death | 0 | 3 (1.3) | – | 0.997 | – | 0.944 |
| TVMI | 8 (12.3) | 11 (4.9) | 2.71 (1.04-7.04) | 0.041 | 2.57 (0.56-6.89) | 0.227 |
| PMI | 8 (12.3) | 10 (4.5) | 2.99 (1.13-7.92) | 0.028 | 2.73 (0.79-6.61) | 0.091 |
| TVR | 1 (1.5) | 0 | – | 0.995 | – | 0.994 |
| ST | 0 | 2 (0.9) | – | 0.997 | – | 0.994 |
| At 1 year | ||||||
| TVF | 16 (24.6) | 15 (6.7) | 4.53 (2.09-9.78) | <0.001 | 4.02 (1.77-6.83) | 0.004 |
| Cardiac death | 1 (1.5) | 3 (1.3) | 1.15 (0.12-11.21) | 0.907 | 1.02 (0.10-1.12) | 0.638 |
| TVMI | 10 (15.4) | 11 (4.9) | 3.50 (1.42-8.67) | 0.007 | 3.35 (1.03-7.33) | 0.045 |
| TVR | 8 (12.3) | 3 (1.3) | 10.29 (2.65-40.04) | 0.001 | 7.95 (1.13-35.98) | 0.037 |
| ST | 0 | 3 (1.3) | – | 0.997 | – | 0.995 |
| Any death | 2 (3.1) | 5 (2.2) | 1.38 (0.26-7.31) | 0.702 | 1.26 (0.21-5.45) | 0.608 |
| At 3 years | ||||||
| TVF | 19 (29.2) | 24 (10.8) | 3.43 (1.73-6.77) | <0.001 | 2.45 (1.39-5.54) | 0.003 |
| Cardiac death | 4 (6.2) | 4 (1.8) | 3.59 (0.87-14.77) | 0.077 | 1.02 (0.09-3.20) | 0.987 |
| TVMI | 11 (16.9) | 12 (5.4) | 3.58 (1.49-8.56) | 0.004 | 3.29 (1.15-6.09) | 0.030 |
| TVR | 10 (15.4) | 5 (2.2) | 7.93 (2.60-24.14) | <0.001 | 6.39 (2.04-13.48) | 0.007 |
| ST | 2 (3.1) | 4 (1.8) | 1.74 (0.31-9.71) | 0.529 | 1.48 (0.26-4.23) | 0.390 |
| Any death | 6 (9.2) | 13 (5.8) | 1.64 (0.59-4.51) | 0.335 | 1.58 (0.46-4.01) | 0.995 |
| Data are n(%). Parameters for adjusted analysis included age, history of percutaneous coronary intervention, peripheral artery disease, renal dysfunction, heart failure, triple-vessel disease, lesion length in the main vessel and side branch, baseline diameter stenosis at the ostial side branch, number of lesions, number of treated lesions, staged percutaneous coronary intervention, final two-stent techniques, and complete revascularisation. CI: confidence interval; HR: hazard ratio; MI: myocardial infarction; PMI: periprocedural myocardial infarction; μQFR: novel quantitative flow ratio; SB: side branch; ST: stent thrombosis; TVF: target vessel failure; TVMI: target vessel myocardial infarction; TVR: target vessel revascularisation | ||||||
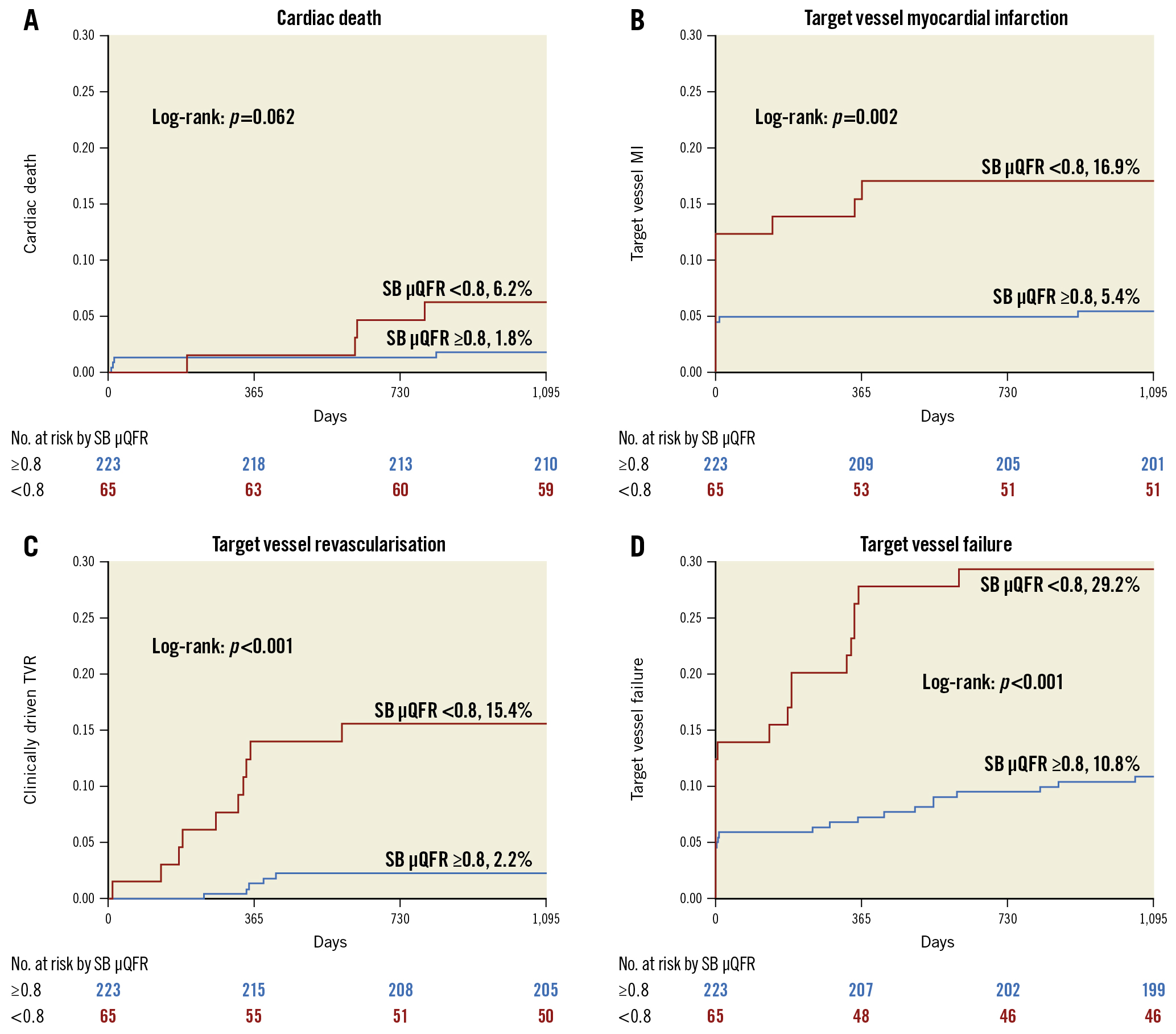
Figure 2. Comparison of primary and secondary endpoints. Comparison of A) cardiac death, B) target vessel myocardial infarction, C) target vessel revascularisation, and D) target vessel failure between patients with a quantitative flow ratio in the SB (SB μQFR) <0.8 and ≥0.8. μQFR: novel quantitative flow ratio; SB: side branch
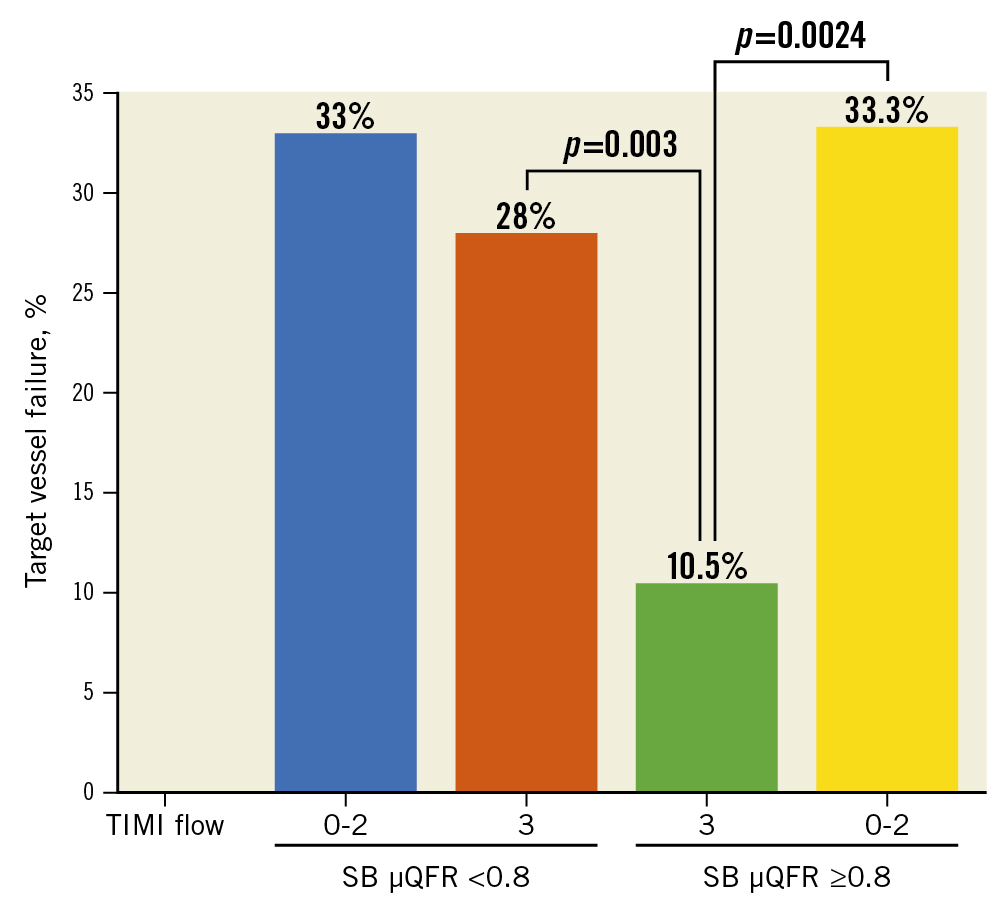
Figure 3. Correlation of SB μQFR with TIMI flow and TVF. SB: side branch; TIMI: Thrombolysis in Myocardial Infarction; TVF: target vessel failure; μQFR: novel quantitative flow ratio
Correlation of SB μQFR <0.8 with SB TIMI flow and TVF
Immediately after the procedures, SB TIMI flow grade <3 was seen in 18 (6.3%) patients, with 15 (23.1%) in the SB μQFR <0.8 group and 3 (1.3%) in the SB μQFR ≥0.8 group (p<0.001) (Table 2). Of patients with SB TIMI flow grade <3, >33.0% of patients suffered 3-year TVF (Figure 4), with no significant difference between the SB μQFR <0.8 and ≥0.8 groups. Notably, of 270 patients with TIMI flow grade 3, the rate of 3-year TVF was 28.0% (14/50) in patients with an SB μQFR <0.8 and 10.5% (23/220) in patients with an SB μQFR ≥0.8 (p=0.003) (Figure 3).
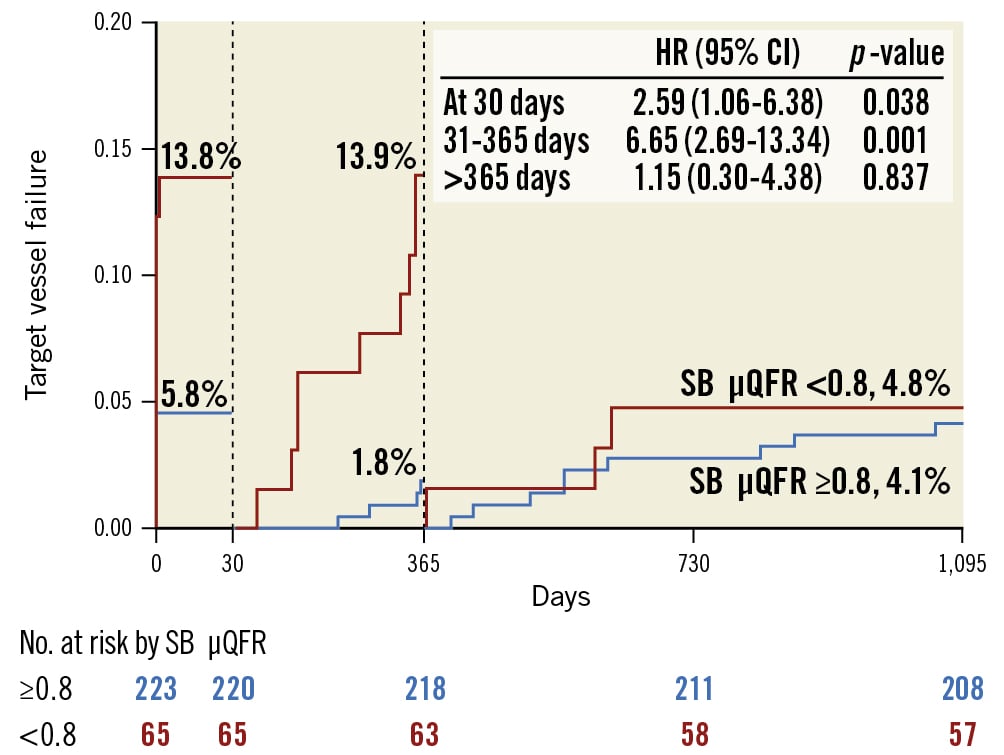
Figure 4. Landmark analysis of target vessel failure. Landmark analysis showed a significant difference in target vessel failure at 30 days and between 31 and 365 days, but not between 366 days and 3 years, for patients with an SB μQFR <0.8 and an SB μQFR ≥0.8. CI: confidence interval; HR: hazard ratio; SB: side branch; μQFR: novel quantitative flow ratio
Discussion
To the best of our knowledge, this is the first study to report the clinical predictive value of the μQFR from a single projection in patients with true coronary artery bifurcation lesions treated with the provisional approach. We successfully measured the μQFR in the MV and SB in 82.7% of patients and found that 1) baseline diameter stenosis at the ostial SB and SB lesion length are predictors of an SB μQFR <0.8 immediately after stenting the MV; 2) a post-procedural SB μQFR <0.8 is strongly associated with TVMI, TVR, and subsequent TVF, within one year of the procedure; 3) previous PCI and an SB μQFR <0.8 predict the occurrence of three-year TVF.
The measurement of the μQFR has the advantage of not requiring the administration of adenosine (which may induce some side effects, i.e., dyspnoea and bradyarrhythmia) or the use of a costly pressure wire, and subsequently shortens the measuring time (usually 1-2 min)6. Since μQFR measurements rely largely on the identification of arterial boundaries from angiography, the reproducibility of μQFR is a major issue. Kornowski et al7 reported that a high degree of concordance was found between two measurements of QFR performed by two different operators (interclass correlation coefficient of 0.97; p<0.001), which is consistent with our results. Since angiographic quality is the determinant of a successfully measured QFR789101112131415161718, the failure of measurements varied from 5.9%10 to 16%9, similar to our findings. The common feature of these lesions is slow flow, with a QFR>0.80 due to no significant stenosis, but a TIMI flow grade <3. It is important to note that the μQFR only assesses the presence of ischaemia caused by lesions in the epicardial coronary vessels, so coronary microvascular lesions may present with a normal QFR but slow blood flow. Recently, a meta-analysis19 of 16 studies demonstrated that 18% of evaluated vessels could not be analysed. Obviously, prospective analysis will promote the successful measurement of QFR given that the quality of angiography meets the requirements19. Diagnostic performance of the QFR is another concern. Results from the WIFI II Study10 showed that the overall sensitivity, specificity, and positive and negative predictive values of the QFR in a single vessel were 77%, 86%, 75%, and 87%, respectively. The sensitivity and specificity of the QFR increased to 86.5% and 88.9%, respectively, in the FAVOR II China study12, in line with a recent pooled analysis19. However, the accuracy of the SB μQFR was not analysed because of a lack of wire-based FFR in the SB.
Coronary bifurcation lesions account for 20-25% of treated lesions12. Although a 3D model of a bifurcated vessel for QFR measurement was introduced in 201520, successful measurement of the μQFR in both the MV and SB using a single projection was only reported more recently14 in 330 vessels in the FAVOR II China study15. The vessel-based analysis demonstrated that not only had the sensitivity remained stable, but the specificity had increased from 89%19 to 96.2%14. Contrary to the FAVOR II China study15, our study included all bifurcation lesions needing treatment and reported a much lower baseline μQFR for both the MV and SB. Another important finding was the MV μQFR <0.89 after the stenting procedure, which was found in 124 (15.1%) patients, similar to the 13% of 123 vessels with suboptimal results from the HAWKEYE study9. Using wire-based FFR after stenting the MV, an SB FFR <0.75 was seen in 26% of 110 patients with bifurcation lesions21, similar to the 22.6% in our study, using a cut-off of 0.8 for the μQFR.
Post-stenting wire-based FFR was the major predictor of clinical events after bifurcation stenting421222324. Unfortunately, while QFR has generally been accepted to be an alternative to functional parameters for ischaemia, there is no study systematically analysing the association of SB μQFR after the provisional approach with clinical outcomes. At 30 days after the procedure in our study, the differences in TVF and TVMI (driven by periprocedural MI) between the SB μQFR <0.8 and ≥0.8 groups by unadjusted analysis became non-significant after the adjusted analysis. However, the significant differences in TVMI, TVR, and TVF were sustained through three years of follow-up by either unadjusted or adjusted analyses (Table 4). Furthermore, landmark analysis failed to show the difference in TVF from one year to three years between the two groups. Consequently, the more solid correlation of an SB μQFR <0.8 with the occurrence of TVMI, TVR, and TVF within one year has emerged, as most TVFs took place within one year.
The next concern is how to predict QFR in SBs after stenting the MV. The reasons for a reduced FFR post-stenting are multifactorial and include the presence of a muscle bridge, distal lesions, spasm, and microcirculatory dysfunction. In this study, age and SB lesion length were predictors of an SB μQFR <0.8 after the MV intervention. As a result, measurement of the μQFR in the SB after stenting the MV should be recommended, particularly for bifurcation lesions with long lesion lengths in the SB. We also found that previous PCI and an SB μQFR <0.8 were the two independent factors for TVF at three-year follow-up. The important role of SB lesion length has been clearly studied in the DEFINITION study25, in which a lesion length ≥10 mm in the SB was the major criterion for defining complex bifurcation lesions. The recently published DEFINITION II trial2 further confirmed the superiority of the systematic two-stent approach to the provisional approach for bifurcation lesions with complex coronary bifurcation lesions. Another striking finding was that an SB μQFR <0.8 was not rare (6.3%) among patients with SB TIMI flow grade 3; however, the underlying mechanisms may be correlated with microcirculatory dysfunction. Altogether, routine measurement of the μQFR in the SB after stenting the MV should be performed, particularly for lesions with a long lesion length in the SB.
Limitations
This study has several limitations. First, the coronary angiographies in the study were obtained without adherence to a dedicated QFR acquisition protocol; therefore, the QFR could not be analysed in 17.3% of the lesions, which hampered a per-patient and intention-to-treat analysis. The relatively high exclusion rate shows, in our opinion, that the quality of the image matters and supports the theory that there are optimal postures to expose lesions and improve measurement accuracy. Second, only patients with bifurcation lesions treated with the provisional approach were selected, which constituted a selection bias and did not allow for calculation of the real rate of SB μQFR <0.8 after both two- and one-stent techniques; however, this study aimed to analyse the impact of the SB μQFR on clinical outcomes for bifurcation lesions treated with provisional stenting only. Third, intravascular imaging was used in fewer than 35% of lesions. This may have increased the number of μQFR <0.89 in the MV and the likelihood of μQFR <0.8 in the SB. Therefore, translating our data into clinical practice should be done very cautiously. Fourth, as a post hoc analysis of μQFR measurements, patients with a reduced SB μQFR could not be randomly studied. Finally, the SB μQFR was not compared with pressure wire-based FFR. It is known that not only SB stenosis but also bifurcation angles and the amount of myocardium subtended contribute to FFR values in the SB. Therefore, further elaboration on the potential impact of these factors on the SB μQFR would be of interest. But our finding has linked the SB μQFR to clinical outcomes, which means the SB μQFR is clinically relevant. Altogether, further study is required to analyse the accuracy of the SB μQFR and to compare the treatment effects of two-stent vs one-stent techniques for an SB μQFR <0.8 after stenting the MV.
Conclusions
The μQFR can be reliably measured in most patients with coronary bifurcation lesions. An SB μQFR <0.8 is strongly correlated with clinical events.
Impact on daily practice
In coronary bifurcations, the novel μQFR derived from a single angiographic projection has an acceptable performance as compared to wire-based FFR. However, the relationship between the side branch (SB) μQFR and clinical outcomes after provisional stenting is unclear. In this study, we found a strong correlation between an SB μQFR <0.8 and target vessel failure within one year of MV stenting procedures. SB lesion length plays an important role in predicting the SB μQFR and clinical events after stenting the MV. The μQFR should be routinely measured and used to guide the necessity of SB treatment.
Acknowledgements
We deeply acknowledge Dr Bao-Xiang Duan (Chair) as the director of the independent committee. We thank Ms Ling Lin and Ms Hai-Mei Xu for their contributions to the completion of this study. Moreover, we appreciate Ms Lingling Liu, Ms Rong-Fang Wang, Ms Wen Teng, and Ms Yingying Zhao for remote monitoring and data collection throughout the study. We also appreciate the support of the Cooperative Innovational Center of Nanjing Medical University throughout the study.
Funding
The trial was funded by a grant from the National Science Foundation of China (Funding no.: NSFC 91639303, NSFC 81770441), Jiangsu Provincial Special Program of Medical Science, and jointly supported by the Nanjing Municipal Medical Development Project.
Conflict of interest statement
The authors have no conflicts of interest to declare.

