Introduction
Lifestyle changes have escalated the prevalence of coronary artery disease (CAD) widely, especially in middle-aged and elderly patients. Percutaneous coronary intervention (PCI) has been considered the gold standard for coronary artery revascularisation for the last five decades1. There have been various approaches to treat obstructive lesions in coronary arteries, which are characterised by the deposition of plaque in the lumen of these vessels, in turn, restricting the myocardial blood flow. Tremendous advances in PCI technologies have taken place over decades from transluminal-only coronary balloon angioplasty to recent drug-eluting stents (DES) with bioresorbable polymers1. Stent platform technology has grown from bare metal stents (BMS) to series of newer-generation DES. The first-generation DES aimed to reduce the risk of stent thrombosis (ST) along with the repeated revascularisation and restenosis rates2. The second-generation DES further addressed these issues. However, the second-generation DES were also associated with a higher risk of major adverse cardiac events (MACE), ST and late restenosis in some cases, and the plausible reason was often ascribed to the thicker strut size. This has thus fuelled the need for thinner struts and advanced generations of DES12. Research has showed that by reducing the strut thickness to a minimum, the rate of MACE and ST can fall to near zero3. Also, replacing the durable polymer with biodegradable polymers like poly lactic-co-glycolic acid (PLGA) and poly-L-lactic acid (PLLA) may reduce stimulation of the inflammatory response4. This led to the conceptualisation of Evermine50 everolimus-eluting stent (EES; Meril Life Sciences Pvt. Ltd). It is the world’s thinnest-strut (50 μm) EES with a biodegradable-polymer coating. It has potential advantages over thicker struts, because it causes less vessel injury, inflammation, and thrombus formation, and enables faster endothelialisation and early vascular healing, with a lower risk of malapposed struts being observed45. Clinical outcomes from various studies showed that Evermine50 EES was a safer and more reliable stent to treat long and multiple lesions345.
A single-centre retrospective study in 171 patients showed no procedural complications and no definite or probable ST with a relatively low all-cause mortality-to-survivorship ratio4. On the other hand, a prospective study in 711 subjects treated with >40 mm Evermine50 EES revealed a lower rate of the device-oriented composite endpoint (6.6%), which was a composite of cardiac death, target vessel myocardial infarction (TVMI) and clinically driven target lesion revascularisation (CD-TLR) at 12 months, and further showed a lower rate of definite and probable ST of 1.1% and 0.8%, respectively, in patients with unduly long lesions at 12-month follow-up6.
In this present prospective, single-arm, post-marketing multicentre study, we report our experience on the safety and performance of the ultrathin-strut Evermine50 EES in patients suffering from de novo coronary artery lesions.
Methods
STUDY DESIGN AND POPULATION
This prospective, single-arm, multicentre (9 centres), post-marketing study conducted over a 24-month period evaluated the safety and performance of Evermine50 EES implanted in patients suffering from de novo coronary artery lesions. Patients above 18 years of age were enrolled in the study. Patients with hypersensitivity to heparin, cobalt-chromium (CoCr) metal alloy, everolimus, polymer lactide, glycolide, and antiplatelet drugs, like prasugrel, clopidogrel etc., and those who did not provide written informed consent were not included in the study.
DEVICE DESCRIPTION AND PROCEDURAL DETAILS
The Evermine50 EES is the world’s thinnest (50 μm) everolimus-eluting stent. It has a biodegradable polymer, PLGA- and PLLA-coated EES system, developed on the CoCr platform. The device is European Conformity (CE)-marked and commercially available in varied lengths and diameters7.
PROCEDURAL AND POSTINTERVENTIONAL MEDICATIONS
The PCI procedure was performed as per the American College of Cardiology/American Heart Association guidelines8. Preprocedure, all the patients were given 75-100 mg aspirin and 300 mg of clopidogrel or as per the investigators’ discretion. Heparin (70-100 units/kg) was used to maintain an activated coagulation time >250 seconds as per the requirements of the PCI procedure.
STUDY ENDPOINTS
The safety endpoint was MACE (which was a composite of cardiac death, myocardial infarction [MI], and CD-TLR) and stent thrombosis at 1-, 6-, 12- and 24-month follow-up. The performance endpoint comprised ischaemia-driven target lesion revascularisation (ID-TLR), ischaemia-driven target vessel revascularisation (ID-TVR) up to 24 months, and procedural (within 24 hours) and device success (post-procedure). In-stent and in-segment minimal lumen diameter (MLD) and late lumen loss (LLL) at 12-month follow-up were compared to pre- and post-PCI by quantitative coronary angiography (QCA) as assessed at an independent core lab (CBCC Global Research LLP, Ahmedabad, India).
STATISTICAL ANALYSIS
The demographic and baseline characteristics are summarised using descriptive statistics. Categorical variables are described as counts and percentages and were compared using Pearson’s chi-square test or Fisher’s exact test. Continuous variables are described as means±standard deviation (SD) and were compared using the t-test or Mann-Whitney U test, as appropriate. A 2-sided p-value of less than 0.05 was considered to indicate statistical significance. All statistical analyses were performed using Statistical Package for the Social Sciences software, version 22 (IBM).
Results
BASELINE DEMOGRAPHIC CHARACTERISTICS
A total of 121 patients were screened, of whom 3 failed the screening test and 118 were enrolled in the study (Figure 1). The mean age of the patients was 58.16±10.73 years, and the majority were males (80.51%) (Table 1). Out of the 118 patients, 64 (54.24%) had single-vessel disease, followed by 39 (33.05%) and 15 (12.71%) patients with double- and triple-vessel disease, respectively (Figure 2). The medical histories of the 118 enrolled patients were studied; 38.98% had diabetes, 47.46% were suffering from hypertension, and 36.44% were smokers (Figure 3).
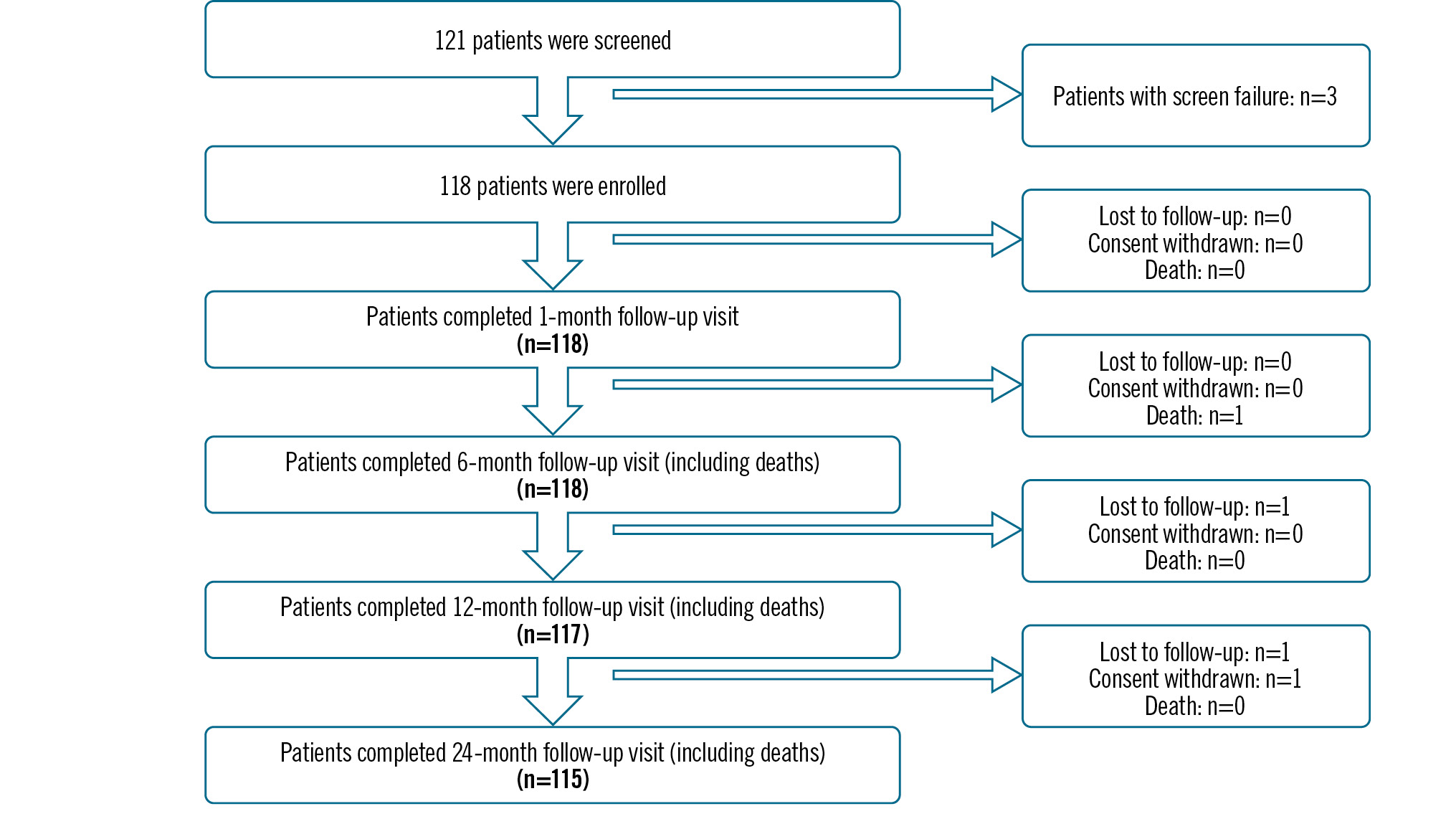
Figure 1. CONSORT flow diagram for 24-month follow-up.
Table 1. Baseline characteristics and demographic details.
| Variables | n=118 patients |
|---|---|
| Age, years | 58.16±10.73 |
| Sex | |
| Male | 95 (80.51) |
| Female | 23 (19.49) |
| BMI*, kg/m2 | 24.90±3.70 |
| Heart rate, bpm | 77.58±11.04 |
| DBP, mmHg | 80.21±11.64 |
| SBP, mmHg | 127.25±18.34 |
| Values are mean±standard deviation or n (%). *n=116. BMI: body mass index; bpm: beats per minute; DBP: diastolic blood pressure; SBP: systolic blood pressure | |
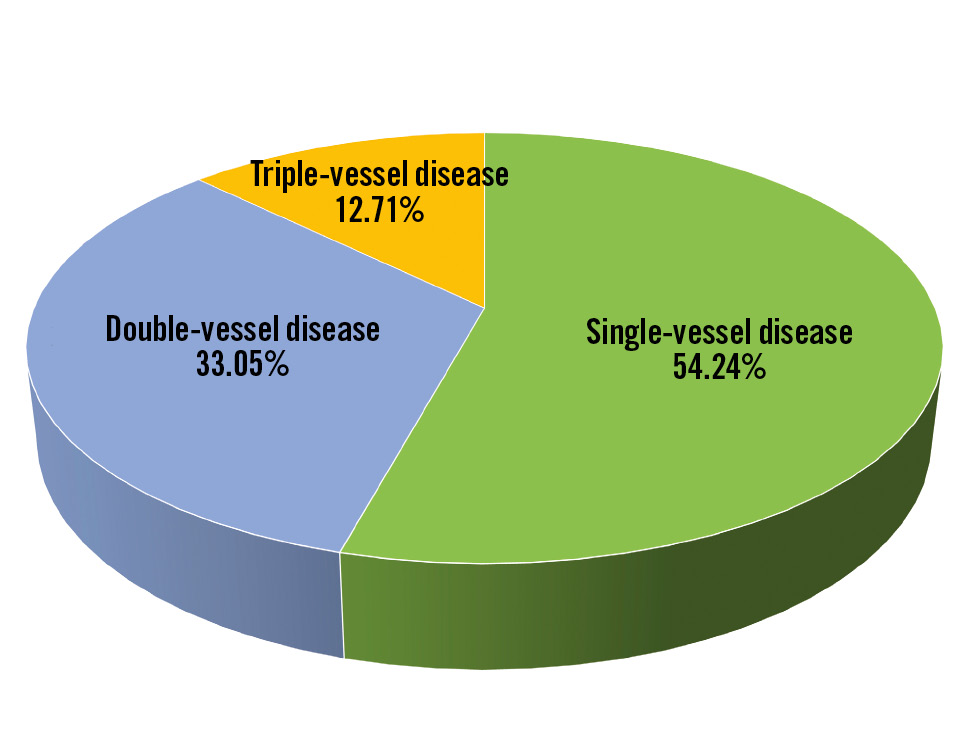
Figure 2. Distribution of the diseased vessels.
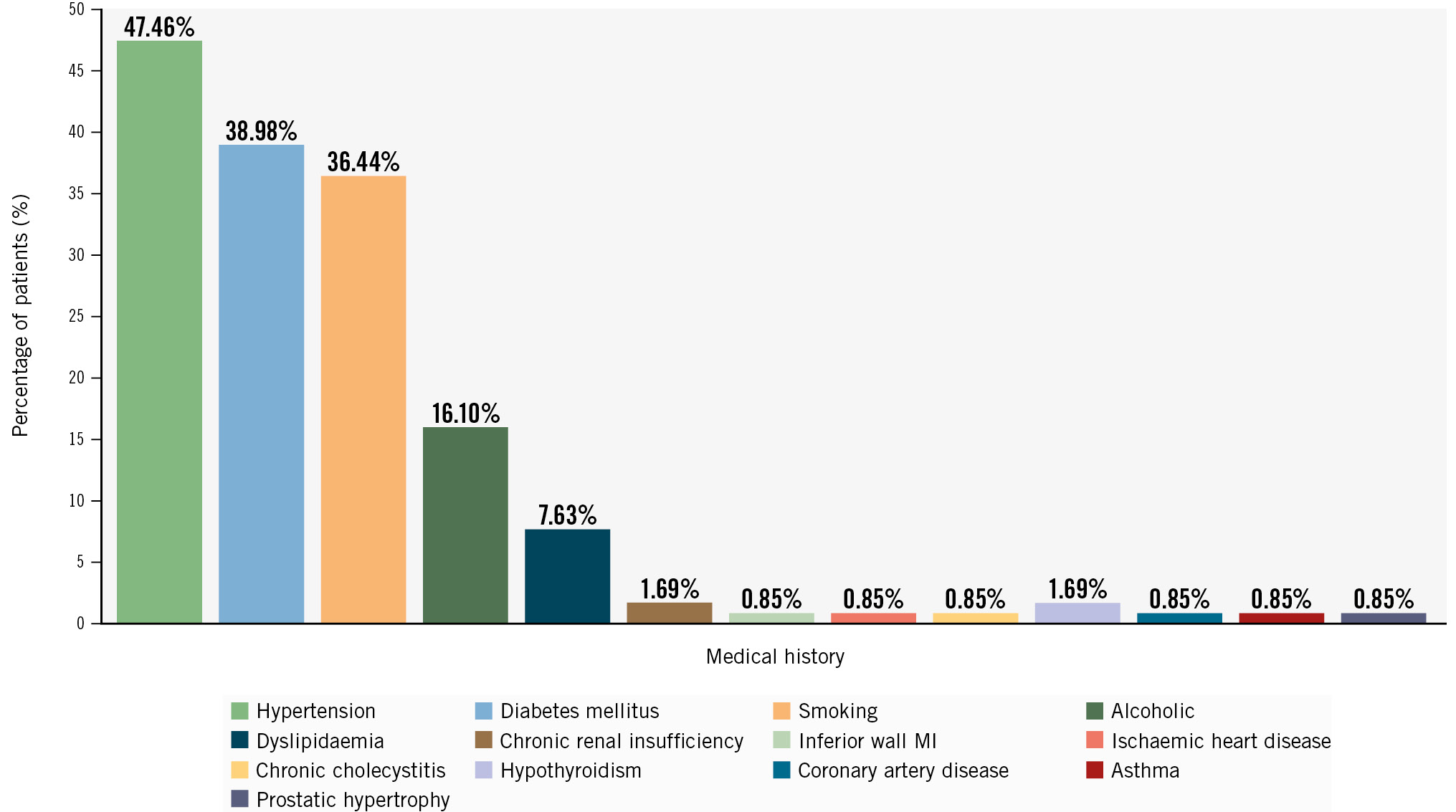
Figure 3. Medical and cardiac history of the patients. MI: myocardial infarction
LESION CHARACTERISTICS
A total of 138 lesions were treated in 118 patients. The most common target vessel treated was the left anterior descending artery, in 63 (45.65%) patients, followed by the right coronary artery, in 46 (33.33%) patients, and the left circumflex artery, in 19 (13.77%) patients (Table 2). Predilatation was performed in 66.19% of patients, while post-dilatation was performed in 77.70% of patients. The average diameter and length of the implanted Evermine50 EES were 2.97±0.36 mm (range: 2.25-3.50 mm) and 26.02±9.24 mm (range: 13-48 mm), respectively. In 34.75% of the patients, the right femoral artery was the access point; in the remaining patients, the right or left radial artery was used as the access site (Table 2). A large proportion of patients (40.29%) had preprocedural Thrombolysis in Myocardial Infarction (TIMI) flow grade 0, which improved post-procedure to TIMI flow grade 3 in 99.28% of patients (Table 2). Acute procedural success, defined as TIMI flow grade 3, and device success, defined as successful implantation of the device, were found to be 100%, with no MACE nor any adverse events during the index procedure (Table 2).
Table 2. Lesion and procedural characteristics.
| Variables | 118 patients/ 138 lesions |
|---|---|
| Lesion characteristics | |
| Total number of lesions treated with study device | 138 |
| Total number of study stents deployed | 139* |
| Number of lesions per patient | 1.17 |
| Lesion location | |
| RCA | 46 (33.33) |
| LAD | 63 (45.65) |
| LCx | 19 (13.77) |
| OM1 | 7 (5.07) |
| LPDA | 1 (0.72) |
| Ramus | 1 (0.72) |
| LMCA | 1 (0.72) |
| ACC/AHA lesion types | n=125 |
| Type A | 49 (39.2) |
| Type B1 | 42 (33.6) |
| Type B2 | 5 (4.0) |
| Type C | 29 (23.2) |
| Average stent length, mm | 26.02±9.24 |
| Average stent diameter, mm | 2.97±0.36 |
| Diameter, mm | |
| 2.25 | 2 (1.44) |
| 2.50 | 23 (16.55) |
| 2.75 | 36 (25.90) |
| 3.00 | 41 (29.50) |
| 3.50 | 37 (26.62) |
| Length, mm | |
| 13 | 9 (6.47) |
| 16 | 22 (15.83) |
| 19 | 21 (15.11) |
| 24 | 29 (20.86) |
| 29 | 14 (10.07) |
| 32 | 16 (11.51) |
| 37 | 7 (5.04) |
| 40 | 15 (10.79) |
| 44 | 3 (2.16) |
| 48 | 3 (2.16) |
| TIMI flow preprocedure | |
| 0 | 56 (40.29) |
| 1 | 28 (20.14) |
| 2 | 55 (39.57) |
| 3 | 0 (0) |
| TIMI flow post-procedure | |
| 0 | 0 (0) |
| 1 | 0 (0) |
| 2 | 1 (0.72) |
| 3 | 138 (99.28) |
| Predilatation | 92 (66.19) |
| Post-dilatation | 108 (77.70) |
| LVEF, % | 50.06±9.65 |
| Diameter stenosis, % | 87.43±11.38 |
| Procedure access site | |
| Femoral – right | 41 (34.75) |
| Radial – right | 75 (63.56) |
| Radial – left | 2 (1.69) |
| Contrast media used | |
| Ionic | 20 (16.95) |
| Non-ionic | 98 (83.05) |
| Procedural success | 118 (100) |
| Device success | 118 (100) |
| Values are n, n (%) or mean±standard deviation. *One lesion was treated with two Evermine50 EES (Meril Life Sciences Pvt. Ltd). ACC: American College of Cardiology; AHA: American Heart Association; EES: everolimus-eluting stent; LAD: left anterior descending artery; LCx: left circumflex artery; LMCA: left main coronary artery; LPDA: left posterior descending artery; LVEF: left ventricular ejection fraction; OM1: first obtuse marginal; RCA: right coronary artery; TIMI: Thrombolysis in Myocardial Infarction | |
CLINICAL OUTCOMES
At the end of the 24-month follow-up period, 1 (0.87%) patient experienced MACE. None of the patients exhibited MI, ID-TLR, ID-TVR, CD-TLR or ST during the 24 months of follow-up. There was 1 all-cause death reported during the 6-month follow-up period, which was a cardiac death (due to cardiorespiratory arrest). Detailed clinical outcomes are shown in Table 3. Myocardial reperfusion was re-established to TIMI flow grade 3 in 99.28% of patients post-PCI, while TIMI flow grade 2 was achieved in 0.72% of patients (Table 3).
Table 3. Cumulative clinical events up to 24-month follow-up.
| Clinical events | Post-procedure | 1 month | 6 months | 12 months# | 24 months$@ |
|---|---|---|---|---|---|
| n=118 | n=118 | n=118 | n=117 | n=115 | |
| All-cause death | 0 (0) | 0 (0) | 1 (0.85) | 1 (0.85) | 1 (0.87) |
| Cardiac death | 0 (0) | 0 (0) | 1 (0.85) | 1 (0.85) | 1 (0.87) |
| Non-cardiac death | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| MI | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| CD-TLR | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ID-TLR | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ID-TVR | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ST | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| MACE | 0 (0) | 0 (0) | 1 (0.85) | 1 (0.85) | 1 (0.87) |
| Values are n (%). #1 patient was lost to follow-up at 12 months; $1 additional patient was lost to follow-up at 24 months; @1 patient withdrew consent at 24-month follow-up. CD-TLR: clinically driven target lesion revascularisation; ID-TLR: ischaemia-driven target lesion revascularisation; ID-TVR: ischaemia-driven target vessel revascularisation; MACE: major adverse cardiac events; MI: myocardial infarction; ST: stent thrombosis | |||||
ANGIOGRAPHIC OUTCOMES
Of 118 patients enrolled in the study, angiographic follow-up was carried out in a subset of patients (n=21) at a mean follow-up of 12 months. An independent core laboratory (CBCC Global Research LLP, Ahmedabad, India), assessed the coronary angiograms preprocedure, post-procedure and at a mean follow-up of 12 months. As per the independent core laboratory’s assessment, the in-device MLD post-procedure and at follow-up were 2.54±0.34 mm and 2.37±0.32 mm, respectively, and the in-segment MLD were 2.41±0.37 mm and 2.28±0.37 mm, respectively, with a p-value<0.0001, as mentioned in Table 4. In-device and in-segment LLL at the mean follow-up (12 months) were 0.17±0.31 mm and 0.12±0.31 mm, respectively. An overview of the Evermine50 EES-1 study is shown in the Central illustration.
Table 4. Quantitative coronary angiography analysis by an independent core lab at a mean follow-up of 12 months.
| Parameters | Preprocedure (n=21) | Post-procedure (n=21) | 12-month mean follow-up (n=21) | p-value (preprocedure vs 12-month follow-up) |
|---|---|---|---|---|
| In-device | ||||
| MLD (mm) | 0.50±0.43 | 2.54±0.34 | 2.37±0.32 | <0.0001 |
| LLL (mm) | – | – | 0.17±0.31 | – |
| In-segment | ||||
| MLD (mm) | 0.50±0.43 | 2.41±0.37 | 2.28±0.37 | <0.0001 |
| LLL (mm) | – | – | 0.12±0.31 | – |
| Values are mean±SD. LLL: late lumen loss; MLD: minimal lumen diameter; SD: standard deviation | ||||
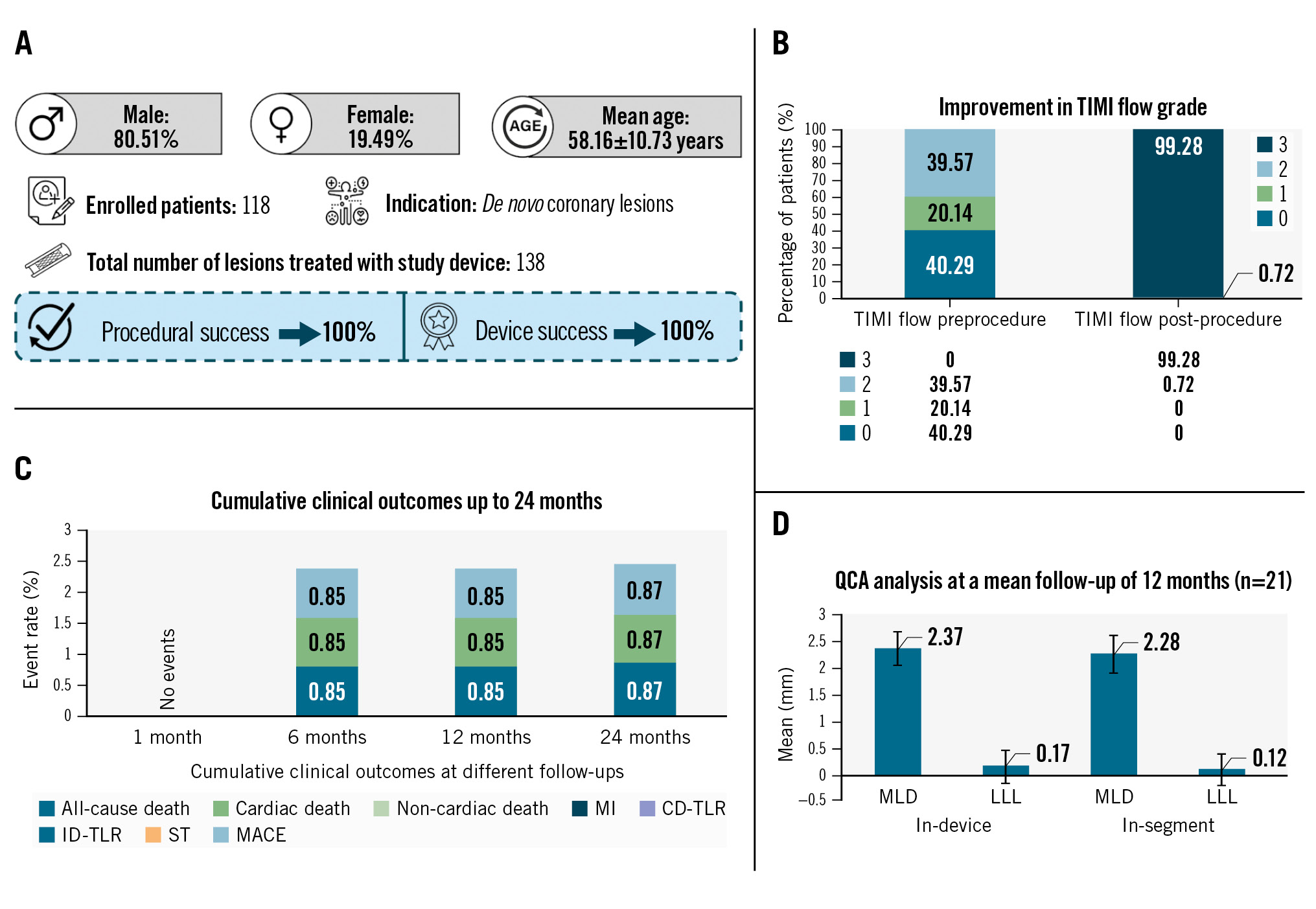
Central illustration. Evermine50 EES-1 study: results from the 24-month follow-up. Over 24 months, satisfactory results were obtained for all patients in terms of safety and performance of the ultrathin Evermine50 EES with a low MACE rate and absence of MI, CD-TLR, ID-TLR, ID-TVR and ST events. A) Demographic and procedural details; B) pre- and postprocedural TIMI flow grades; C) clinical outcomes up to 24-month follow-up; D) QCA analysis at a mean follow-up of 12 months. CD-TLR: clinically driven target lesion revascularisation; EES: everolimus-eluting stent; ID-TLR: ischemia-driven target lesion revascularisation; ID-TVR: ischemia-driven target vessel revascularisation; LLL: late lumen loss; MACE: major adverse cardiac events; MI: myocardial infarction; MLD: minimal lumen diameter; QCA: quantitative coronary angiography; ST: stent thrombosis; TIMI: Thrombolysis in Myocardial Infarction
Discussion
The results obtained from this study can be reported as satisfactory for all the patients in terms of the safety and performance of the Evermine50 EES. Across the demographics and varied medical history of patients, it was observed that there was 100% procedural and device success.
Given that ST − occurring for various reasons such as delayed endothelialisation, hypersensitivity reactions, chronic inflammation of arteries, etc. − has been a major cause of rehospitalisation, this study focused on minimising ST and attained 100% clinical success in preventing ST. ST has always been reported in a higher number of patients implanted with an early-generation DES as compared to BMS. Evermine50 EES, in contrast, has the thinnest strut thickness (50 μm) of currently available devices, which allows streamlining of the flow of blood, minimising platelet aggregation around the stent, and thus reducing the chances of ST5.
In a retrospective study on the treatment of very long coronary artery stenoses (>30 mm) by Patra et al 2020, the Evermine50 EES was compared with the Tetrilimus (Sahajanand Medical Technologies Limited), and it was observed that the results reported for the Evermine50 EES were comparable to those with the Tetrilimus in terms of MACE, MI, ST, and target lesion revascularisation (TLR), and all-cause mortality was approximately 3% in both groups, with zero reports of MI, ST and TLR5.
Shiomi et al 2019 reported the outcomes of the RESET trial, where they compared outcomes of CYPHER SELECT Plus (Cordis), a first-generation sirolimus-eluting stent (SES), with those of XIENCE V (Abbott), a second-generation EES, up to 7 years9. Although target vessel revascularisation (TVR) was not significantly different between the two groups (11.7% for SES and 10.2% for EES), the second-generation EES provided a lower risk of ST than the first-generation SES. The authors concluded that the second-generation EES could provide longer-term improved safety compared to the first-generation SES. Moreover, Panoulas et al 2015 analysed the inception and evolution of various EES platforms and emphasised the superior efficacy and safety of EES over BMS and DES such as paclitaxel-eluting stents and SES10
Picard et al 2019, in a systematic review and meta-analysis, examined and compared the safety and efficacy of biodegradable-polymer EES with durable-polymer DES11. A total of 4,631 patients were included in the analysis, of whom 2,315 patients were included in the biodegradable-polymer EES group and 2,316 patients in the durable-polymer DES group (1,143 patients were treated with an EES and 1,173 patients were treated with a zotarolimus-eluting stent). The baseline characteristics of the patients were similar in both groups except for the higher prevalence of prior MI in the durable-polymer DES group than the biodegradable-polymer EES group (25.7% vs 22.5%; p=0.001). Similarly, the procedural characteristics were also comparable except for the slightly longer lesion length in the biodegradable-polymer EES group compared to the durable-polymer DES group (15.1 mm vs 14.9 mm; p=0.04). No other significant differences were observed in clinical outcomes – cardiac mortality, MI, any TLR, ST or any MACE. The TALENT trial enrolled 1,435 patients to compare the safety and efficacy of Supraflex (an SES with ultrathin struts; Sahajanand Medical Technologies Ltd.) with XIENCE up to 3-year follow-up12. The study confirmed the safety and efficacy of the Supraflex stent as equal to those of the XIENCE stent. On the other hand, the CASTLE trial was conducted to compare an ultrathin biodegradable-polymer SES (BP-SES) with a thin durable-polymer EES (DP-EES) for a 1-year follow-up period13. The study demonstrated that DP-EES was non-inferior to BP-SES based on target lesion failure, and the strut thickness differences among DES have a minimal effect on clinical outcomes.
The BIOSCIENCE randomised trial was conducted to compare the ultrathin (60 μm) BP-SES (Orsiro) and the DP-EES (XIENCE Prime/Xpedition [Abbott]) and reported all-cause mortality for the SES and EES to be 14.1% and 10.3%, respectively. Hence, results were found to be better for ultrathin BP-SES14. In another study, Jiménez et al compared the 80 μm biodegradable-polymer Ultimaster SES (Terumo) with a XIENCE EES, and they found no significant differences between the two devices in terms of cardiac death, ST and MI15. Similar results are reported in the present study, where the absence of ST, MI, CD-TLR, ID-TLR and ID-TVR was observed. This implies that newer stents with thinner struts improve clinical outcomes in patients suffering from multiple and complex lesions. Moreover, Evermine50 EES showed an absence of MI, CD-TLR and a lower rate of cardiac death, which thereby resulted in a lower MACE rate, compared to previous studies on other devices, proving the safety and performance of the ultrathin-strut EES stent. There was significant improvement in MLD from preprocedure to mean follow-up (in device: 0.50±0.43 mm vs 2.37±0.32 mm) with an acceptable range of LLL.
Limitations
Our study has a few limitations; the first is the lack of optical coherence tomography analysis data for all patients due to a small patient pool at the sites. However, QCA was performed at a mean follow-up of 12 months in order to assess the LLL. Secondly, this is a single-arm study with 24 months of follow-up. Further long-term randomised controlled trials including a larger patient population with complex lesion types are needed to compare the Evermine50 EES with other contemporary DES in real-world populations.
Conclusions
This long-term (24-month) follow-up study reiterates that the Evermine50 EES has favourable safety and performance in treating real-world CAD patients suffering from long and multiple lesions, showing improved TIMI flow and low MACE with an absence of ST, ID-TLR, ID-TVR and CD-TLR.
Impact on daily practice
The second-generation drug-eluting stents (DES) were associated with a higher risk of major adverse cardiac events, stent thrombosis and late restenosis in some cases, and the possible reason was often ascribed to the thicker strut size. Thus emerged the need for thinner struts and an advanced generation of DES. Research showed that reducing the strut thickness to a minimum can minimise the adverse outcomes. The midterm outcomes of this everolimus-eluting stent will impact the practice of cardiologists in treating long and multiple de novo coronary artery lesions.
Funding
The Evermine50 EES-1 study was funded by Meril Life Sciences Pvt. Ltd, Gujarat, India.
Conflict of interest statement
A. Thakkar is a full-time employee of Meril Life Sciences Pvt. Ltd. The other authors have no conflicts of interest to declare.

