Ching-Wei Lee1,2, MD; Shih-Hsien Sung1,2*, MD, PhD; Wei-Ming Huang1,2, MD; Yi-Lin Tsai1,2, MD; Chiao-Po Hsu2,3, MD, PhD; Chun-Che Shih2,3, MD, PhD
AsiaIntervention 2019;5:64-67, DOI: 10.4244/AIJ-D-18-00018
1. Division of Cardiology, Department of Medicine, Cardiovascular Research Center, National Yang-Ming University, Taipei, Taiwan; 2. Cardiovascular Research Center, National Yang-Ming University, Taipei, Taiwan; 3. Division of Cardiovascular Surgery, Department of Surgery, Cardiovascular Research Center, National Yang-Ming University, Taipei, Taiwan
Abstract
Percutaneous edge-to-edge mitral repair with the MitraClip device is an alternative therapy for patients with severe mitral regurgitation. Given that Barlow’s disease is characterised by multiple prolapsed segments and multiple regurgitant jets, the MitraClip is not recommended. Herein, we present the case of a 42-yearold gentleman who suffered acute biventricular failure due to a primary chordae rupture of Barlow’s mitral valve. Because of prohibitive surgical risk, he was successfully rescued using transcatheter edge-to-edge mitral repair. Our critical case may demonstrate the feasibility of MitraClip use as a rescue therapy for patients with acute severe mitral regurgitation.
Abbreviations
LAD: left anterior descending artery
MR: mitral regurgitation
MVA: mitral valve area
PEEP: positive end-expiratory pressure
TEE: transoesophageal echocardiography
Introduction
Clinical trials have demonstrated the efficacy and safety of transcatheter edge-to-edge mitral valve repair, using the MitraClip® device (Abbott Vascular, Santa Clara, CA, USA), as an alternative therapy for severe mitral regurgitation (MR). However, anatomical eligibility for this procedure is critically important. Barlow’s disease is characterised by an abnormal matrix structure and multiple leaflet scallops prolapsing into the left atrium. The major limitation of the MitraClip procedure in treating multiple flail segments is the residual mitral valve area (MVA) after the repair. As a result, patients with Barlow’s disease are considered inappropriate for the MitraClip procedure1. On the other hand, open heart surgery may sometimes not be feasible for patients with acute severe MR and prohibitive risks. The case here presented with inoperable Barlow’s disease was finally successfully rescued using the MitraClip procedure.
Methods
A 42-year-old gentleman with Marfan’s syndrome, who had undergone Bentall’s operation at the age of 32, presented to the emergency department with acute pulmonary oedema and was soon intubated due to respiratory failure. Transoesophageal echocardiography (TEE) elucidated multiple MR jets, arising from both the flail A3 leaflet and the prolapsed P1 leaflet (Figure 1A-Figure 1C). The mitral regurgitant volume was 195 ml by the flow convergence method. Otherwise, the billowing bileaflets and marked dilatation of the mitral annulus were typical presentations of Barlow’s mitral valve disease. The transthoracic echocardiogram showed a left ventricular ejection fraction (LVEF) of 45% and a right ventricular fractional area change (FAC) of 25%. In addition, the blood tests disclosed marked elevations of aspartate aminotransferase (2,228 u/l), N-terminal prohormone brain natriuretic peptide (4,350 pg/ml) and serum lactate level (48.5 mg/dl), indicating acute biventricular failure and cardiogenic shock. He was put on high-dose dopamine of 10 mcg/kg/min. Right heart catheterisation demonstrated a mean pulmonary artery wedge pressure of 26 mmHg, a giant V-wave pressure of 33 mmHg, mean pulmonary artery pressure of 39 mmHg, and right atrial pressure of 18 mmHg. The coronary angiogram disclosed a critical stenosis at the proximal portion of the left anterior descending artery (LAD). Given that the calculated EuroSCORE II risk was 60% for surgery, transcatheter intervention was the preferred rescue option through the shared decision-making process. Since the patient did not improve after percutaneous coronary intervention to the LAD, a MitraClip procedure was conducted 48 hours later.
The procedure was conducted using the standard approach with a classic MitraClip device. Due to the higher coaptation point from the prolapse/flail and medial location, we aimed at a higher puncture site of the interatrial septum. The transseptal height from the annulus was 4.4 cm. Continuous left atrial pressure monitoring with a pigtail catheter in the left upper pulmonary vein was performed to assess the immediate haemodynamic response throughout the procedure2. We failed to grasp directly at the largest gap of the flail A3 leaflet and therefore applied the anchoring strategy from the medial commissure. With the increasing of positive end-expiratory pressure (PEEP) to 10 cm H2O, the first clip was applied at the medial site of the A3-P3, followed by additional clips at the lateral site of the A3-P3 and a third clip over the P1 prolapse segment (Figure 1D, Figure 1E). Only trivial to mild residual MR and a transmitral mean pressure gradient of 3 mmHg remained. Before withdrawal of the steerable guide to the left atrium, a right to left shunt from the iatrogenic septal defect was observed by TEE (Moving image 1). Arterial blood gas analysis showed a significant drop of the oxygenation after removal of the steerable guide. The PaO2/FiO2 ratio significantly reduced to 49% as compared with the start of the procedure (372 preoperatively and 184 after removal of the guide). We therefore closed the septal defect with a 12 mm AMPLATZER™ atrial septal defect occluder (St. Jude Medical, St. Paul, MN, USA) (Figure 1F). The vascular access was closed with a “figure-of-eight” suture.
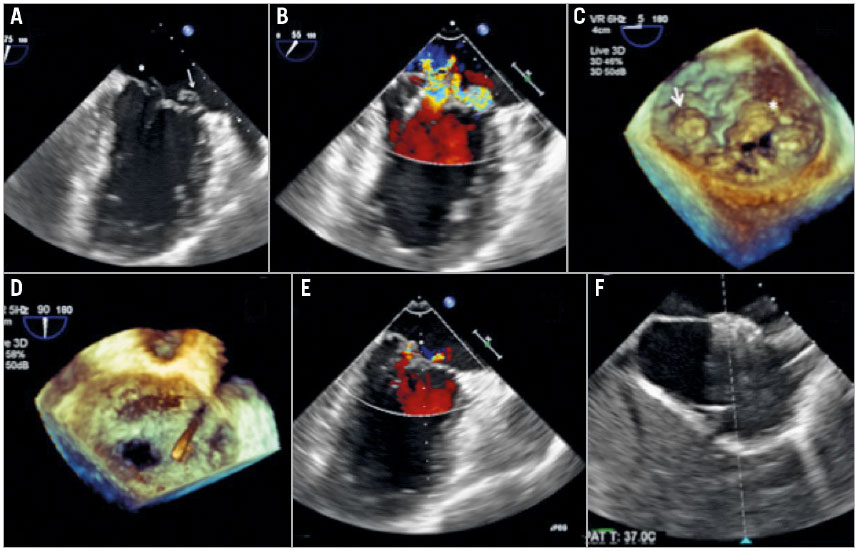
Figure 1. Transoesophageal echocardiography images during the MitraClip procedure. A) Bi-commissural view of the transoesophageal echocardiogram demonstrating the complexity of the Barlow’s mitral valve. Arrow marks the medial A3 flail segment and the asterisk marks the P1 prolapse segment of the mitral valve. B) Bi-commissural view with colour Doppler demonstrating the significant mitral regurgitation arising from both the lateral and the medial site of the mitral valve. C) Three-dimensional en face view elucidating the complexity of the Barlow’s mitral valve. Arrow marks the medial A3 flail segment and the asterisk marks the P1 prolapse segment of the mitral valve. D) Three-dimensional en face view demonstrating a total of three clips successfully implanted. E) Bi-commissural view of the transoesophageal echocardiogram showing only trivial mitral regurgitation after the procedure. F) The iatrogenic atrial septal defect was closed by an AMPLATZER atrial septal defect occluder.
Results
The patient recovered well, and was extubated on the next day and then discharged in a week. At the three-month visit he was asymptomatic, and the transthoracic echocardiogram showed mild MR and normally functioning ventricles.
Discussion
To the best of our knowledge, this is the first report of a MitraClip procedure as the rescue therapy for a Marfan’s patient with Barlow’s mitral valve disease. Barlow’s disease is the most complex form of degenerative mitral valve disease due to the extensive leaflet thickening, multiple segment prolapsing, elongated and ruptured chordae tendineae, and annular dilatation. It is challenging to conduct a MitraClip procedure for Barlow’s disease because the anatomical eligibility is more rigorous than for a central flail leaflet. The huge volume of prolapsing leaflet and poor chordae support make the clipping process more difficult and even warrant multiple clips to stabilise the coaptation. The two most useful techniques to complete this procedure are the anchoring clip strategy2 and applying PEEP to improve coaptation3. With growing experience and newer-generation devices, i.e., MitraClip NT or XTR, mitral cleft4, large flail gap5, or even papillary muscle rupture6 can also be treated by the transcatheter procedure, only if the residual MVA is sufficient. Furthermore, Asians have smaller hearts than Westerners, which makes the MitraClip procedure more challenging in respect of adequate residual MVA7. The presented case had a native MVA of 5.6 cm2 and a residual MVA of 3.2 cm2 after three clips. Even though multiple clips are usually required in Barlow’s disease, the risk of small residual MVA is counterbalanced by the dilated annulus. However, surgical correction of Barlow’s mitral insufficiency should be the first-line therapy since its long-term efficacy has been well documented8.
Limitations
Although over 50% of the patients who have undergone MitraClip implantation have a persistent septal defect for more than six months9, it was not usual to develop an interatrial right-to-left shunt after clipping. The right-to-left or bidirectional shunt may cause systemic hypoxaemia and warrant immediate closure10,11. The presence of the interatrial right-to-left shunt was related to the elevated right atrial pressure. In addition, a 12 mm AMPLATZER atrial septal defect occluder is usually suggested for the closure of an iatrogenic defect12.
Conclusions
While surgical mitral repair is the default treatment for severe MR in patients with Barlow’s disease, MitraClip implantation can be a viable alternative if patients are at prohibitive surgical risk.
Impact on daily practice
Patients with Barlow’s mitral valve disease are considered unfavourable candidates for MitraClip procedures. Surgical correction of the complex anatomy is the gold standard in patients with Barlow’s mitral insufficiency. However, MitraClip implantation can serve as a rescue therapy in critical Barlow’s patients who carry a prohibitive surgical risk.
Funding
This work was supported by a grant from the Taipei Veterans General Hospital, Taiwan, R.O.C. (V106B-019).
Supplementary data
Moving image 1. Transoesophageal echocardiographic image of Barlow’s mitral valve with severe mitral regurgitation.
Conflict of interest statement
The authors have no conflicts of interest to declare.
References
1. Boekstegers P, Hausleiter J, Baldus S, von Bardeleben RS, Beucher H, Butter C, Franzen O, Hoffmann R, Ince H, Kuck KH, Rudolph V, Schäfer U, Schillinger W, Wunderlich N; Germany Society of Cardiology Working Group on Interventional Cardiology Focus Group on Interventional Mitral Valve Therapy. Percutaneous interventional mitral regurgitation treatment using the Mitra-Clip system. Clin Res Cardiol. 2014;103:85-96.
2. Singh GD, Smith TW, Rogers JH. Multi-MitraClip therapy for severe degenerative mitral regurgitation: “anchor” technique for extremely flail segments. Catheter Cardiovasc Interv. 2015;86: 339-46.
3. Patzelt J, Zhang Y, Seizer P, Magunia H, Henning A, Riemlova V, Patzelt TA, Hansen M, Haap M, Riessen R, Lausberg H, Walker T, Reutershan J, Schlensak C, Grasshoff C, Simon DI, Rosenberger P, Schreieck J, Gawaz M, Langer HF. Effects of Mechanical Ventilation on Heart Geometry and Mitral Valve Leaflet Coaptation During Percutaneous Edge-to-Edge Mitral Valve Repair. JACC Cardiovasc Interv. 2016;9:151-9.
4. Lee CW, Sung SH, Chang TY, Tsai IL, Hsu CP, Shih CC. Grasping the Pseudo-Cleft in the Case of a Small, Severely Tethered Posterior Mitral Leaflet. Korean Circ J. 2017;47:536-7.
5. Mizote I, Schirmer J, Schäfer U. A case of successful Mitraclip implantation in a patient having a large coaptation gap under extracorporeal membrane oxygenation (ECMO). Catheter Cardiovasc Interv. 2018;91:827-30.
6. Bahlmann E, Frerker C, Kreidel F, Thielsen T, Ghanem A, van der Schalk H, Grahn H, Kuck KH. MitraClip implantation after acute ischemic papillary muscle rupture in a patient with prolonged cardiogenic shock. Ann Thorac Surg. 2015;99:e41-2.
7. Daimon M, Watanabe H, Abe Y, Hirata K, Hozumi T, Ishii K, Ito H, Iwakura K, Izumi C, Matsuzaki M, Minagoe S, Abe H, Murata K, Nakatani S, Negishi K, Yoshida K, Tanabe K, Tanaka N, Tokai K, Yoshikawa J; JAMP Study Investigators. Normal values of echocardiographic parameters in relation to age in a healthy Japanese population: the JAMP study. Circ J. 2008;72: 1859-66.
8. Melnitchouk SI, Seeburger J, Kaeding AF, Misfeld M, Mohr FW, Borger MA. Barlow’s mitral valve disease: results of conventional and minimally invasive repair approaches. Ann Cardiothorac Surg. 2013;2:768-73.
9. Schueler R, Öztürk C, Wedekind JA, Werner N, Stockigt F, Mellert F, Nickenig G, Hammerstingl C. Persistence of iatrogenic atrial septal defect after interventional mitral valve repair with the MitraClip system: a note of caution. JACC Cardiovasc Interv. 2015;8:450-9.
10. Huntgeburth M, Müller-Ehmsen J, Baldus S, Rudolph V. Postinterventional iatrogenic atrial septal defect with hemodynamically relevant left-to-right and right-to-left shunt as a complication of successful percutaneous mitral valve repair with the MitraClip. Int J Cardiol. 2013;168:e3-5.
11. Losi MA, Strisciuglio T, Stabile E, Castellano G, de Amicis V, Saccenti A, Maresca G, Santoro C, Izzo R, Barbato E, Esposito G, Trimarco B, Rapacciuolo A. Iatrogenic atrial septal defect (iASD) after MitraClip system delivery: The key role of PaO2/FiO2 ratio in guiding post-procedural iASD closure. Int J Cardiol. 2015;197: 85-6.
12. Saitoh T, Izumo M, Furugen A, Tanaka J, Miyata-Fukuoka Y, Gurudevan SV, Tolstrup K, Siegel RJ, Kar S, Shiota T. Echocardiographic evaluation of iatrogenic atrial septal defect after catheterbased mitral valve clip insertion. Am J Cardiol. 2012;109: 1787-91.
To download, please click below.