Introduction
Transcatheter aortic valve replacement (TAVR) is widely accepted as a definitive therapeutic option for patients with severe aortic stenosis, and its effectiveness has been demonstrated in several randomised trials123456. In recent years, TAVR has demonstrated comparable results to surgical aortic valve replacement, even in surgical low-risk patients78. As a result, the indications for TAVR have expanded to patients with longer life expectancy.
A relatively large number of patients with aortic stenosis have concomitant coronary artery disease9. In previous large trials, patients with aortic stenosis complicated by coronary artery disease comprised 15-81% of the study population12345678. This variation may have been due to differences in the average age of participants. Since coronary artery disease is a progressive disease, the likelihood of developing it increases with age. As the indications for TAVR gradually shift towards younger patients, the need for post-TAVR percutaneous coronary intervention (PCI) will inevitably increase, even if there is no coronary lesion requiring intervention at the time of TAVR.
Following surgical aortic valve replacement, cannulation of the coronary arteries is relatively straightforward because the bioprosthetic valve is implanted to align the neo-commissure with the native commissures. However, it is not always possible to completely align a transcatheter heart valve (THV) with the native commissures in a TAVR procedure. Therefore, the THV neo-commissure is sometimes positioned to face the ostium of the coronary artery10. A prosthesis in the Evolut (Medtronic) series can be placed in a unique supra-annular position, which may further complicate the process of engaging a catheter in cases where the THV neo-commissure faces the coronary artery11.
Aligning the THV neo-commissure with the native commissure can facilitate coronary access after TAVR, especially when using Evolut devices. Therefore, we investigated whether it is possible to implant an Evolut device in a manner that allows access to the coronary artery after TAVR. To do this, we adjusted the direction of the delivery catheter, taking into account the structural characteristics of the Evolut device.
Methods
Study population
In this study, we enrolled patients with severe aortic stenosis who had undergone TAVR with an Evolut device at the Cardiovascular Center of Saiseikai Kumamoto Hospital between March 2019 and April 2022 and who had postoperative electrocardiogram-gated contrast-enhanced cardiac computed tomography (CT) imaging. During the first half of the study period, TAVR was performed using the conventional technique, in which the delivery catheter was advanced normally into the body to the aortic position. All of these patients underwent contrast-enhanced CT at the time of their outpatient visit, unless they met the exclusion criteria. In the latter half, all patients underwent TAVR with adjustment of the delivery catheter direction. Any patients who did not meet the exclusion criteria underwent contrast-enhanced CT during hospitalisation. We excluded the following cases from analysis: 1) TAVR performed with approaches other than the transfemoral approach; 2) TAVR for failed surgical bioprostheses; 3) TAVR for a bicuspid aortic valve; 4) an estimated glomerular filtration rate <30 ml/min/1.73 m2 (increased risk of worsening renal function due to the use of contrast media); 5) lack of patient consent for the use of contrast media (Figure 1).
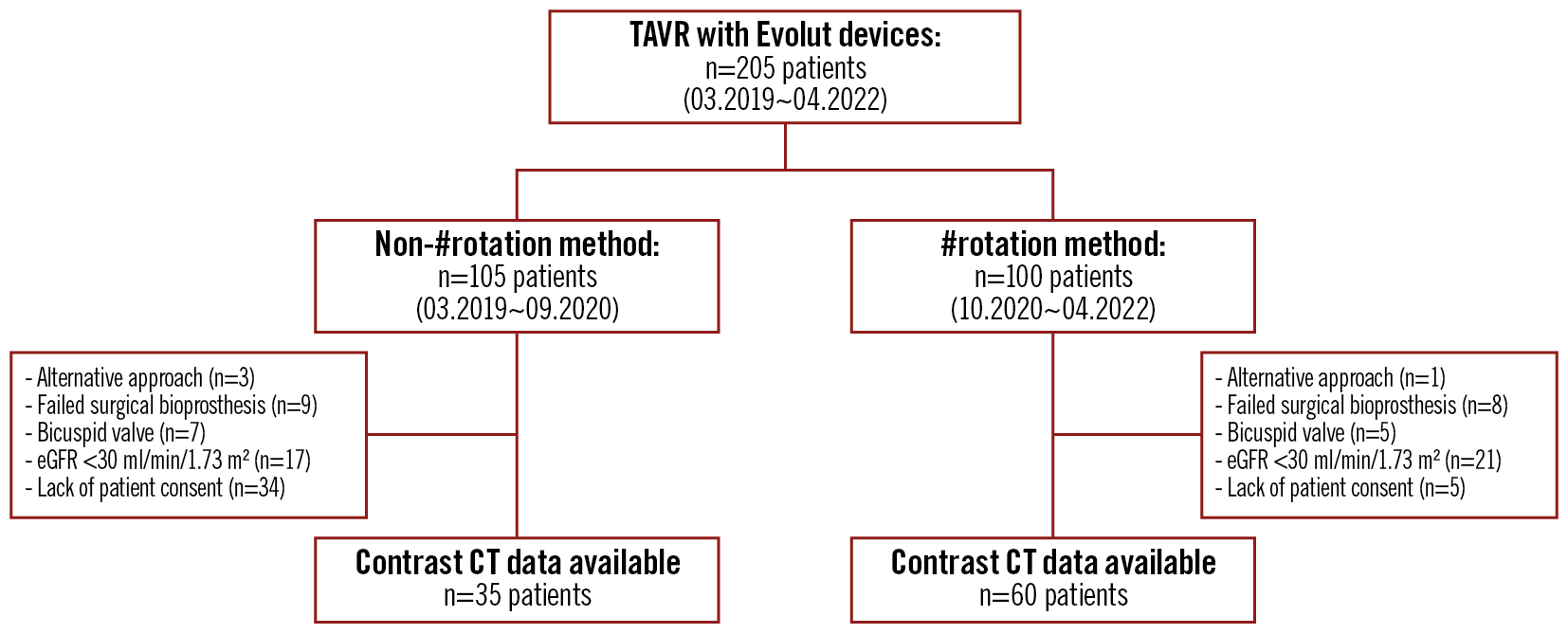
Figure 1. Patient flow diagram and study design. #rotation method: HAt marker-guided Shaft (HASH) rotation method; CT: computed tomography; eGFR: estimated glomerular filtration rate; TAVR: transcatheter aortic valve replacement
Intentional delivery for commissural alignment
The method used to orient the hat marker and place it at the aortic valve was called the HAt marker-guided SHaft (HASH) rotation method (stylised as the #rotation method in this study) (Figure 2). When the delivery system was initially inserted into the body, the flush port was oriented at 3 o’clock. Next, we used the left anterior oblique view to check whether the hat marker was facing the side of the greater curvature of the descending thoracic aorta. If not, the shaft of the delivery system was rotated so that the hat marker faced the side of the greater curvature. This orientation of the hat marker was maintained while the delivery system was passed through the aortic arch to just above the aortic valve. Thereafter, the angle of the fluoroscopy system was changed to the cusp-overlap view of the right and left coronary cusps (RCC/LCC). The THV was implanted after confirming that the hat marker was positioned centre front on the fluoroscopic display. Even though the hat marker was displaced from the centre front position in this location in the aortic valve, rotation of the delivery system in this position was not performed because of concerns about the potential risk of access route injury. We also did not pull the delivery system back to the descending aorta, because we thought it would increase the risk of cerebral infarction. The non-#rotation method in this study was the conventional technique, in which the flush port orientation was at 12 o’clock at insertion and the orientation of the hat marker was unknown after insertion.
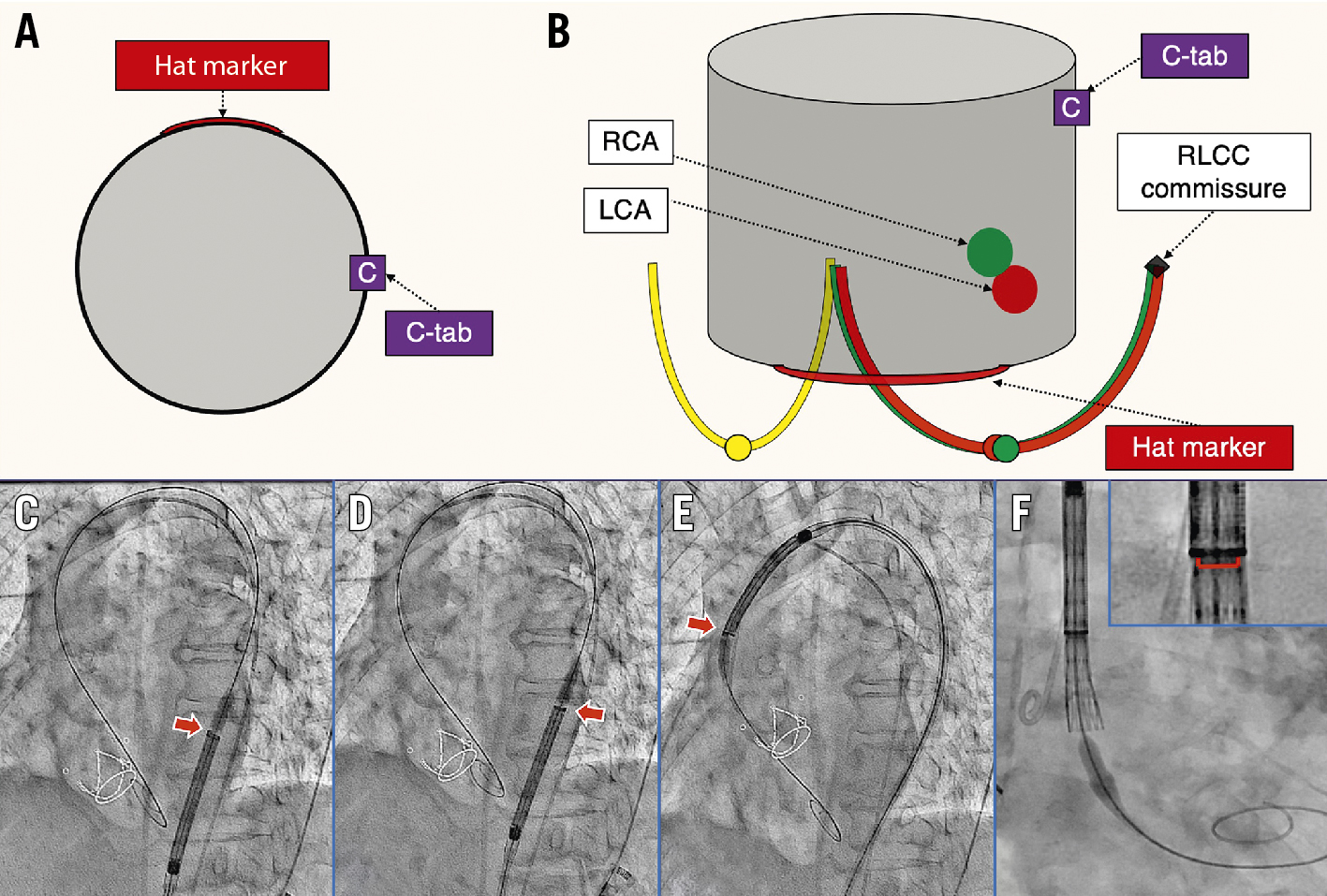
Figure 2. The #rotation method. A) Positional relationship between the hat marker and the C-tab of the THV. B) The aortic valve viewed from the angle of overlap between the RCC (green curve) and LCC (red curve). When the hat marker is located in the centre front position, the C-tab is located near the native RLCC commissure. C,D) The hat marker (red arrow) should face the greater curvature of the descending thoracic aorta. Otherwise, the shaft of the delivery catheter should be rotated. E) If the hat marker passes through the aortic arch facing the greater curvature, it is positioned centre front (red arrow) at the position of the aortic valve (F). #rotation method: HAt marker-guided SHaft (HASH) rotation method; LCA: left coronary artery; LCC: left coronary cusp; RCA: right coronary artery; RCC: right coronary cusp; RLCC: right/left coronary cusp; THV: transcatheter heart valve
Electrocardiogram-gated cardiac CT images
Electrocardiogram-gated cardiac CT was performed with a collimation of 320×0.5 mm. The range of tube current values was automatically set for each patient under model-based iterative reconstruction conditions, and the tube potential was fixed at 100 kV. Images were reconstructed in 0.5 mm thick slices at 0.5 mm intervals with no overlap. Iterative reconstruction was performed with RR intervals of 10% ranging from 0% to 90%. CT images were reconstructed using the 3mensio Valves software (version 9.1; Pie Medical Imaging) and analysed in our dedicated core laboratory. The relationships between the THV neo-commissure and the coronary artery (right: RCA; left: LCA) ostia were evaluated with end-diastolic CT data. First, 3 orthogonal planes were used for multiplanar reconstruction to create a centreline orthogonal to the THV and to locate each THV neo-commissure. The inflow level of each THV was checked, and the distance between the THV inflow and the inferior border of each ostium of the coronary artery was measured. The angle between the native right/left coronary cusp (RLCC) commissure and the C-tab commissure was also evaluated (Figure 3).
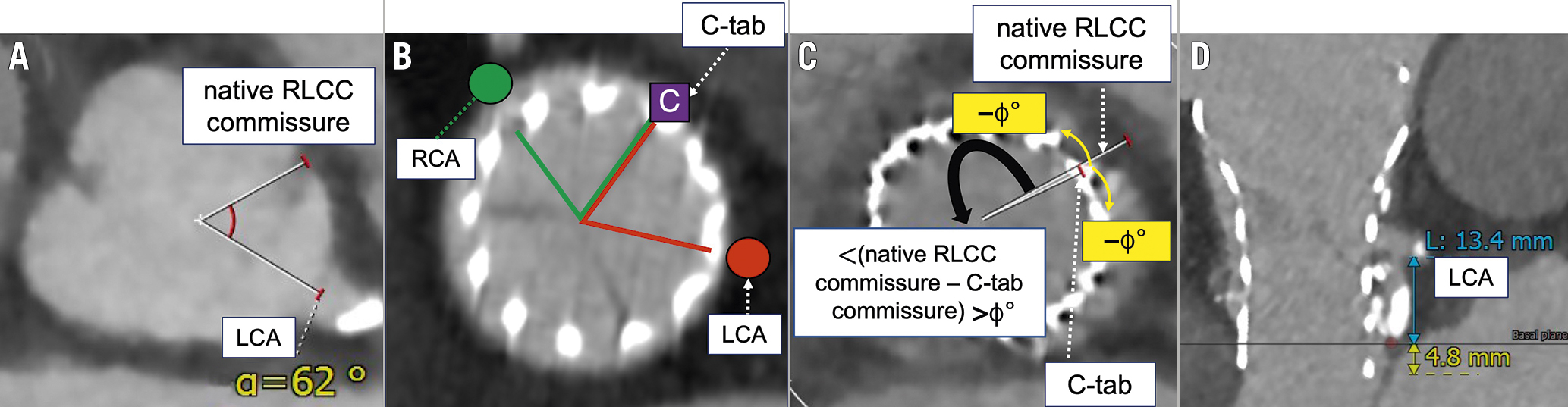
Figure 3. Examples of cardiac computed tomography measurements. Angles between (A) the native RLCC commissure and the LCA; B) the C-tab commissure and the RCA or LCA; and (C) the C-tab commissure and the native RLCC commissure. When the native RLCC commissure is oriented clockwise or counterclockwise relative to the C-tab, the angle is indicated with a positive or negative value, respectively. D) The distance between the THV inflow and the inferior border of the LCA ostium (total length of the blue and yellow lines). LCA: left coronary artery; LCC: left coronary cusp; RCA: right coronary artery; RCC: right coronary cusp; RLCC: right/left coronary cusp; THV: transcatheter heart valve
Study endpoints
The primary endpoint of this study was to orient the THV frames such that they faced the LCA/RCA ostium to a degree that allowed coronary access after TAVR. As a secondary endpoint, we evaluated whether the angle between the C-tab commissure and the LCA/RCA ostium was suitable for coronary access after TAVR. In Evolut devices, the structure of the prosthetic valve varies according to its size. Therefore, favourable angles (relative to the C-tab commissure) for coronary access were defined as 24-96° for the 23 mm valves and 36-84° for valves sized 26, 29, and 34 mm. These estimates accounted for the fact that angles that overlap with the commissural triangles of the THV should be avoided. In this study, we used THV frames without a commissural triangle or skirt.
Ethical statement
The study was conducted according to the principles of the Declaration of Helsinki, and the protocol was approved by the institutional review board or ethics committee of each participating centre. All participating patients gave written/oral consent.
Statistical analysis
Continuous variables were denoted as the mean±standard deviation (SD) or median (interquartile range [IQR]), as appropriate. Categorical variables were reported as numbers and percentages. Continuous variables were compared using the Student’s t-test or Wilcoxon signed-rank sum test, depending on the distribution of the variables. Categorical variables were compared using either the chi-squared test or Fisher’s exact test, depending on the frequencies in the contingency table. The JMP Pro software (version 16; SAS Institute) was used for all statistical analyses. A 2-sided p-value<0.05 was considered to indicate statistical significance.
Results
Data were analysed from a total of 95 patients, including 35 who underwent TAVR with other methods (the non-#rotation group) and 60 who underwent TAVR with the #rotation method. The overall mean age was 84.6 years, and 66.3% of the participants were women, with no significant differences between the 2 groups (Table 1). There were no between-group differences in renal function or history of coronary artery disease. Preoperative transthoracic echocardiography showed that the severity of aortic stenosis was similar across groups.
There were no between-group differences in the size of the THV, fluoroscopy time, or the percentage of patients who underwent TAVR using an in-line sheath (Table 2). In the #rotation method group, 42 patients (70.0%) did not require manual rotation of the delivery catheter to properly orient the hat marker, and 18 patients (30.0%) required manual rotation. Among cases that required rotation, no case presented any difficulty in maintaining the orientation of the hat marker while the delivery system was passed through the aortic arch. The #rotation method did not increase the risk of vascular complications or postoperative cerebral infarction. On the other hand, in the non-#rotation group, only 23 out of 35 patients had a fluoroscopic record that could confirm the orientation of the hat marker as it passed through the aortic arch. Among them, the hat marker faced the greater curvature in 47.8% (11/23 patients).
In the preoperative electrocardiogram-gated cardiac CT, the angles between the native RLCC commissure and the respective coronary artery were 62.5±10.1° (LCA) and 77.0±14.2° (RCA) (Table 3). The height of the coronary artery from the basal annular plane was 12.7±2.5 mm (for the LCA) and 16.0±2.6 mm (for the RCA). There were no significant differences in these anatomical characteristics between the non-#rotation and #rotation groups.
We also compared postoperative electrocardiogram-gated cardiac CT imaging data between the groups (Table 3). The mean angles between the native RLCC and C-tab commissures were not significantly different between groups (non-#rotation: −8.0°; #rotation: 3.0°; p=0.79). However, the C-tab commissure was placed within ±30° of the native RLCC commissure more frequently in the #rotation group than in the non-#rotation group (#rotation: 53 patients, 88.3%; non-#rotation: 13 patients, 42.9%; p<0.001). Similar results were noted when the angle was limited to ±15° (#rotation: 34 patients, 56.7%; non-#rotation: 7 patients, 22.9%; p=0.001). This indicated that C-tab commissures placed with the #rotation method were more closely aligned with the native RLCC commissures (Figure 4).
The angle between the C-tab commissure and the LCA was significantly smaller in the #rotation group than in the non-#rotation group (#rotation: 67.1±20.9°; non-#rotation: 90.2±30.1°; p<0.001). However, there was no between-group difference in the angle of the C-tab commissure relative to the RCA (#rotation: 76.5±23.0°; non-#rotation: 81.9±33.5°; p=0.348). Favourable angles for post-TAVR access to the LCA were significantly more frequent in the #rotation group (#rotation: 55 patients, 91.7%; non-#rotation: 19 patients, 62.9%; p=0.001). For access to the RCA, there was no significant between-group difference in the frequency of favourable angles (#rotation: 50 patients, 83.3%; non-#rotation: 24 patients, 68.6%; p=0.125) (Figure 5).
The distance between the THV inflow and the inferior border of each ostium of the coronary artery was not significantly different between groups in either the LCA or the RCA. To visualise the positional relationship between the THV and the coronary artery, we generated a scatter plot with the angle between the C-tab commissure and the coronary artery on the horizontal axis and the distance between the THV inflow and the inferior border of the ostium of the coronary artery on the vertical axis (Figure 6). The sample size in the group with the 23 mm prosthetic valve was too low for statistical comparisons. Therefore, we compared the data among patients who had received the 26, 29, and 34 mm prosthetic valves. The orientation of the THV frame facilitated post-TAVR access to the LCA more frequently in the #rotation group (#rotation: 52 patients, 91.2%; non-#rotation: 21 patients, 63.6%; p=0.001). However, the #rotation method did not provide such an advantage in the RCA (#rotation: 47 patients, 82.4%; non-#rotation: 24 patients, 72.4%; p=0.34).
Table 1. Baseline clinical and echocardiographic characteristics of patients.
| All(n=95) | #rotation method(n=60) | Non-#rotation method (n=35) | p-value (#rotation method vs non-#rotation method) | |
|---|---|---|---|---|
| Age, years | 84.6±5.1 | 84.8±5.5 | 84.2±4.4 | 0.578 |
| Female | 63 (66.3) | 37 (61.7) | 25 (71.4) | 0.378 |
| BMI, kg/m2 | 22.9±3.2 | 22.5±2.7 | 23.5±3.9 | 0.147 |
| STS score, % | 5.2±2.6 | 5.2±2.6 | 5.2±2.7 | 0.93 |
| eGFR, ml/min/1.73 m2 | 53.9±15.6 | 52.3±16.3 | 56.7±14.0 | 0.181 |
| BNP, pg/dl | 157.0 [55.9, 291.8] | 172.0 [54.9, 372.7] | 122.9 [57.1, 220.9] | 0.241 |
| Coronary artery disease | 23 (24.2) | 17 (28.3) | 6 (17.1) | 0.321 |
| Atrial fibrillation | 18 (18.9) | 13 (21.7) | 5 (14.3) | 0.429 |
| Prior permanent pacemaker | 8 (8.4) | 7 (11.7) | 1 (2.9) | 0.251 |
| LVEF, % | 64.0 [59.5, 68.0] | 63.0 [55.8, 66.0] | 66.0 [64.0, 69.5] | 0.004 |
| Peak aortic velocity, m/s | 4.6±0.6 | 4.6±0.7 | 4.6±0.5 | 0.992 |
| Aortic valve area, cm2 | 0.67±0.16 | 0.66±0.17 | 0.67±0.13 | 0.759 |
| Values are n (%), mean±SD, or median [interquartile range]. #rotation method: HAt marker-guided SHaft (HASH) rotation method; BMI: body mass index; BNP: brain natriuretic peptide; eGFR: estimated glomerular filtration rate; LVEF: left ventricular ejection fraction; SD: standard deviation; STS: Society of Thoracic Surgeons | ||||
Table 2. Procedural characteristics.
| Procedural characteristics | |||||
|---|---|---|---|---|---|
| All(n=95) | #rotation method(n=60) | Non-#rotation method(n=35) | p-value (#rotation method vs non-#rotation method) | ||
| THV size, mm | 23 | 5 (5.3) | 3 (5.0) | 2 (5.7) | 0.406 |
| 26 | 52 (54.7) | 31 (51.7) | 21 (60.0) | ||
| 29 | 33 (34.7) | 21 (35.0) | 12 (34.3) | ||
| 34 | 5 (5.3) | 5 (8.3) | 0 (0) | ||
| Contrast dye, ml | 62.5 [59.0, 85.0] | 57.0 [47.0, 78.5] | 70.0 [57.0, 101.0] | 0.004 | |
| Fluoroscopy time, min | 24.8 [19.9, 28.8] | 24.8 [21.1, 29.3] | 24.9 [18.4, 27.7] | 0.391 | |
| In-line sheath | 85 (89.5) | 55 (91.7) | 30 (85.7) | 0.49 | |
| Rotation of delivery system | 18 (18.9) | 18 (30.0) | 0 (0) | <0.001 | |
| Complications | 18 (18.9) | 8 (13.3) | 10 (28.6) | 0.102 | |
| Vascular complications | 8 (8.4) | 2 (3.3) | 6 (17.1) | 0.048 | |
| Cerebral infarction | 3 (3.2) | 3 (5.0) | 0 (0) | 0.295 | |
| New pacemaker implantation | 8 (8.4) | 4 (6.7) | 4 (11.4) | 0.461 | |
| Values are n (%), mean±SD, or median [interquartile range]. #rotation method: HAt marker-guided SHaft (HASH) rotation method; SD: standard deviation; THV: transcatheter heart valve | |||||
Table 3. Baseline and post-TAVR findings on multislice computed tomography.
| All(n=95) | #rotation method(n=60) | Non-#rotation method(n=35) | p-value (#rotation method vs non-#rotation method) | |
|---|---|---|---|---|
| Baseline MSCT | ||||
| Area, mm2 | 400±65 | 410±68 | 377±53 | 0.027 |
| Perimeter, mm | 72.2±5.7 | 73.1±6.1 | 70.4±4.6 | 0.046 |
| LCA height, mm | 12.7±2.5 | 12.9±2.5 | 12.1±2.2 | 0.133 |
| RCA height, mm | 16.0±2.6 | 16.3±2.6 | 15.3±2.6 | 0.092 |
| <(native RLCC commissure – LCA), ° | 62.5±10.1 | 62.8±10.4 | 62.0±9.5 | 0.728 |
| <(native RLCC commissure – RCA), ° | 77.0±14.2 | 78.6±13.0 | 73.7±16.2 | 0.13 |
| Post-TAVR MSCT | ||||
| THV inflow to LCA ostium | 18.1±3.8 | 18.4±4.0 | 17.7±3.3 | 0.4 |
| THV inflow to RCA ostium | 19.2±4.1 | 19.5±3.7 | 18.6±4.8 | 0.333 |
| <(C-tab commissure – LCA), ° | 75.6±27.0 | 67.1±20.9 | 90.2±30.1 | <0.001 |
| <(C-tab commissure – RCA), ° | 78.5±27.3 | 76.5±23.0 | 81.9±33.5 | 0.348 |
| <(C-tab commissure – native RLCC commissure), ° | 3.0 [-23.0, 16.0] | 3.0 [-16.0, 14.0] | -8.0 [-31.0, 64.5] | 0.796 |
| Values are n (%), mean±SD, or median [interquartile range]. #rotation method: HAt marker-guided SHaft (HASH) rotation method; LCA: left coronary artery; MSCT: multislice computed tomography; RCA: right coronary artery; RLCC: right/left coronary cusp; SD: standard deviation; TAVR: transcatheter aortic valve replacement; THV: transcatheter heart valve | ||||
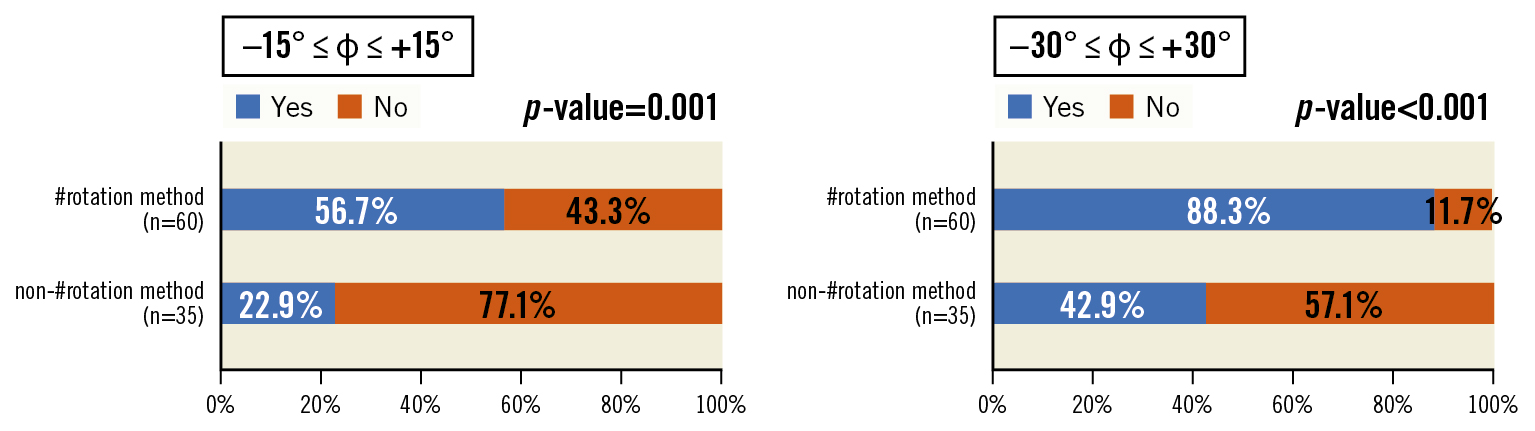
Figure 4. Angles between the native RLCC commissure and C-tab commissure (φ). The percentage of patients in whom the C-tab commissure is placed at ±15° relative to the native RLCC commissure is significantly higher in the #rotation group (56.7%) than in the non-#rotation group (22.9%). The percentage in the #rotation group is even higher (88.3%) when the permitted angle is increased to ±30°. #rotation method: HAt marker-guided SHaft (HASH) rotation method; RLCC: right/left coronary cusp
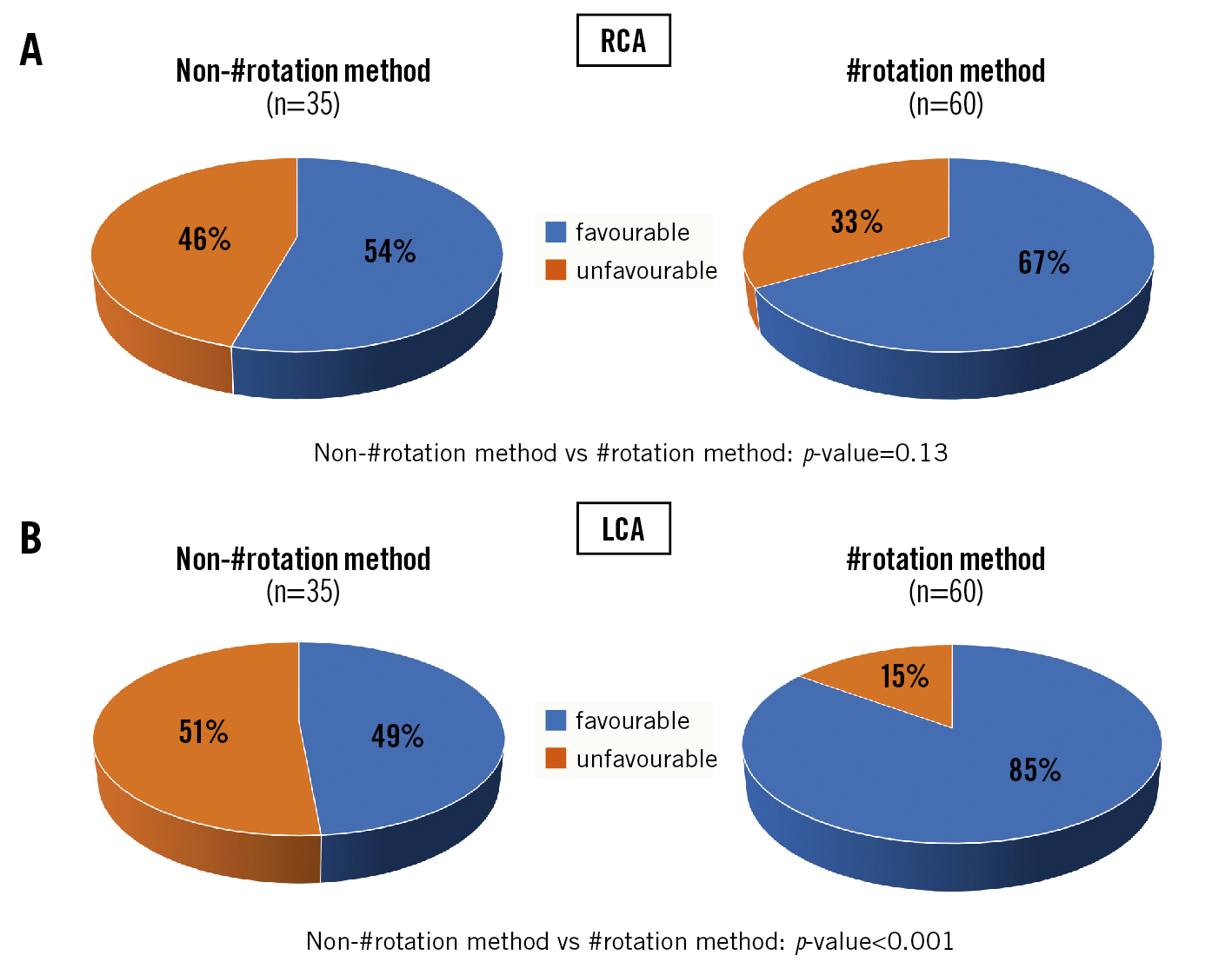
Figure 5. Incidence of favourable angles for post-TAVR coronary access. A) The percentage of patients with a favourable angle between the C-tab commissure and the ostium of the RCA is higher in the #rotation group, but the difference is not significantly significant. B) This difference is statistically significant in the LCA. #rotation method: HAt marker-guided SHaft (HASH) rotation method LCA: left coronary artery; RCA: right coronary artery; TAVR: transcatheter aortic valve replacement
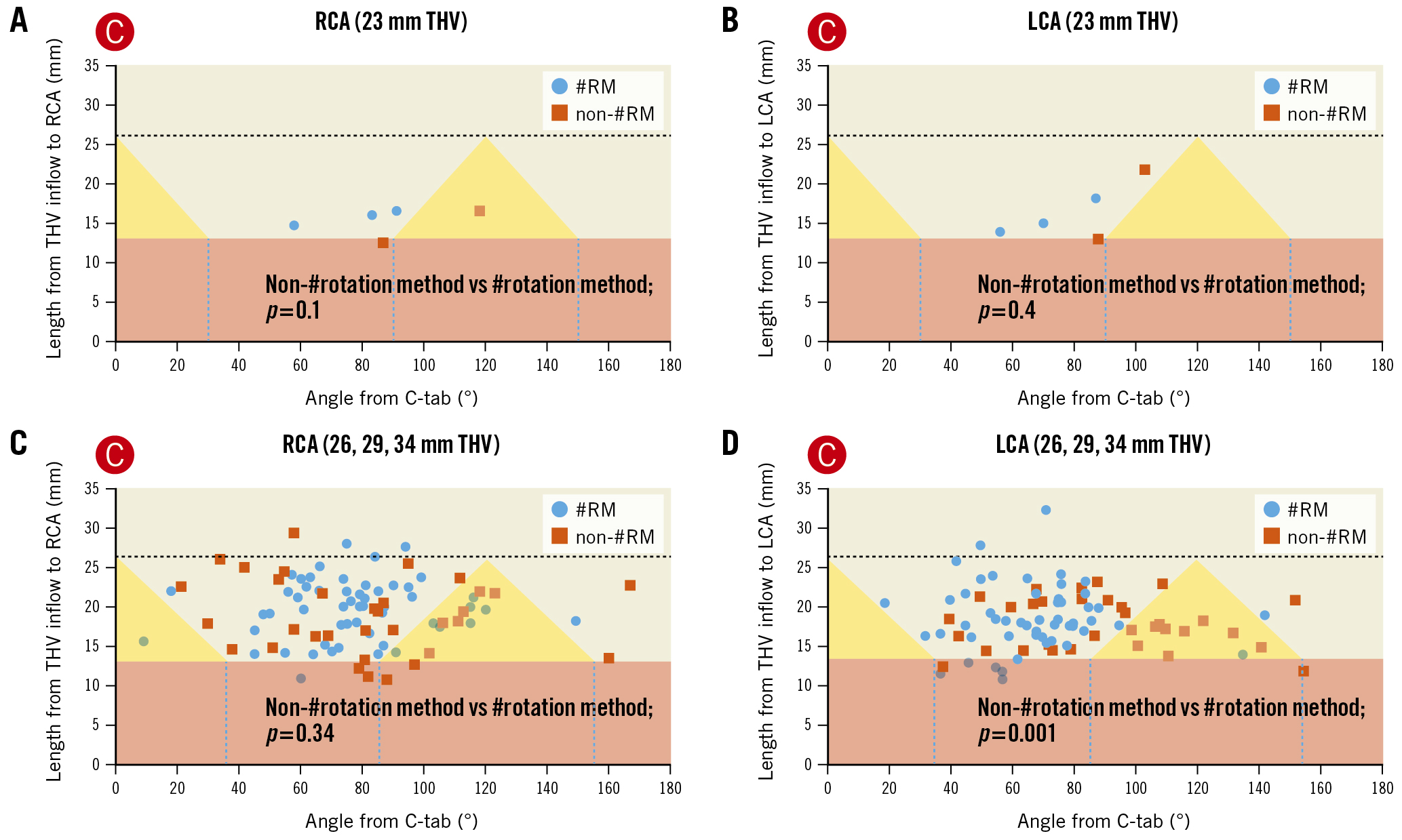
Figure 6. Favourable orientation of THV cells for post-TAVR coronary access. A, B) The x-axes show the angle between the C-tab commissure and the ostium of the coronary artery. The y-axes show the distance between the THV inflow and the ostium of the coronary artery. The pink and yellow areas correspond to the skirt and commissural triangle, respectively. The blue circles and orange squares represent the ostium of the coronary artery in the #rotation and non-#rotation groups, respectively. THVs with a size of 23 mm were excluded from analysis due to a low sample size. C) There is no between-group difference for THVs ≥26 mm with regard to coronary access to the RCA. D) The #rotation method is superior for coronary access to the LCA. #rotation method: HAt marker-guided SHaft (HASH) rotation method; LCA: left coronary artery; RCA: right coronary artery; RM: rotation method; TAVR: transcatheter aortic valve replacement; THV: transcatheter heart valve
Discussion
We investigated whether an Evolut THV can be oriented in a way that facilitates coronary access after TAVR. To achieve this, we adjusted the orientation of the hat marker on the delivery system when positioning it at the aortic valve. The main results of this study are summarised below.
1. Compared with non-#rotation methods, the #rotation method allowed us to place the C-tab commissure closer to the native RLCC commissure.
2. The LCA ostium did not face the commissural triangle of the THV and was positioned at a more favourable angle for coronary artery access in the #rotation group than in the non-#rotation group. In contrast, there was no significant between-group difference in the angle in the RCA.
3. When distances between the THV inflow and the coronary artery ostia were considered in addition to the orientation of the THV, the #rotation group had a higher probability of favourable frame alignment for post-TAVR access to the LCA than the non-#rotation group.
As the indications for TAVR expand to include low-risk and younger patients, the number of patients who require post-TAVR PCI is expected to increase. Although there are limited data regarding the incidence of coronary events after TAVR, acute coronary syndrome after TAVR is known to result in high mortality12. Several factors complicate post-TAVR coronary access, including device-related and procedural factors (such as the orientation of the commissural tab, height of the sealing skirt, and depth of the valve implant) and the patient’s anatomy13. The orientation of the THV is particularly important for coronary access when using Evolut prosthetic valves, as these have a taller frame than other devices14.
Coronary access after THV implantation is a major concern after TAVR and has been the subject of many studies101314151617. Aligning the THV neo-commissure with the native commissure can greatly facilitate coronary access after TAVR18. In Evolut valves, the C-tab paddle is located at a 90° angle clockwise from the hat marker on the delivery catheter19. Since the C-tab paddle coincides with one of the THV neo-commissures, adjusting the hat marker to the proper orientation can enable the proper deployment of the C-tab commissure. Normally, when the hat marker passes through the aortic arch facing the greater curvature of the descending thoracic aorta in the left anterior oblique view, it is also at the centre front in the RCC/LCC cusp-overlap view. In these cases, the native RLCC commissure is located at the left edge of the fluoroscopic display in the RCC/LCC cusp-overlap view. The hat marker being in the centre front indicates that the C-tab commissure is close to the native RLCC commissure.
Tang et al have recommended that the delivery system be inserted with the flush port oriented towards the 3 o’clock position, and the hat marker be tracked to see whether it faces the greater curvature in the aortic arch and is positioned in the centre front of the aortic valve position or not17. Compared with the conventional method, this method has a higher probability of implanting the prosthetic valve in the preferred orientation. However, it is not clear how often the hat marker did not face the greater curvature and whether they rotated the delivery system that way. The vascular tortuosity in the iliac artery and abdominal aorta has an impact on the direction of the hat marker at the aortic arch. Indeed, approximately 30% of the cases in this study required rotation of the delivery system, even though the flush port was inserted in the 3 o’clock position.
Bieliauskas et al reported that adjusting the orientation of the THV based on the fluoroscopic marker on the delivery catheter is useful for neo-commissural alignment20. Our present study differs from this view in two respects. First, the authors evaluated the angle deviations between the native aortic valve commissures and the THV neo-commissures, whereas we examined the angle between the C-tab commissure and the LCA or RCA. Second, they rotated the delivery catheter at the level of the aortic valve, whereas we rotated it at the level of the descending thoracic aorta. The Evolut delivery system has 2 shaft spines that face each other. Therefore, adjusting the orientation of the hat marker after crossing the aortic arch is difficult in terms of operability and is not recommended because of the risk of vascular injury and damage to the delivery catheter21. Therefore, if the orientation of the delivery system needs to be adjusted, this should be done before it passes through the aortic arch. Our results suggest that adjustment at the level of the descending thoracic aorta is appropriate.
In TAVR with Evolut devices, THV positions which are unfavourable for future coronary access are more common in the LCA than in the RCA10. In general, the LCA is a more important myocardial perfusion territory than the RCA. As such, ensuring access to the LCA is essential for long-term prognosis after TAVR. The present study shows that the LCA is located approximately 60° clockwise from the native RLCC commissure. Therefore, placing the C-tab commissure closer to the native RLCC commissure can reduce the risk of the LCA ostium facing the commissural triangle of the THV. Moreover, the LCA tends to be closer to the basal annular plane than the RCA. Considering the presence of the sealing skirt, it is important to have a preoperative plan that adjusts the orientation of the THV in the #rotation method and also places it at a depth that takes into account the height of the coronary artery.
Previous studies have also reported that the success rate of post-TAVR coronary angiography is lower for the RCA than for the LCA22. Unfortunately, the #rotation method failed to demonstrate superiority over the conventional method in facilitating access to the RCA after TAVR. This may be because, compared with the LCA, the RCA is oriented at a larger angle (~80° clockwise) relative to the native RLCC commissure. This makes the RCA more susceptible to the effects of misalignment between the C-tab commissure and the native RLCC commissure. If the C-tab commissure could be aligned with the RCA-LCA-centred line instead of the native RLCC commissure, it would be more favourable for access to both coronary arteries. However, it is technically difficult to align with the RCA-LCA-centred line at present. Furthermore, it is generally known that the RCA ostium is more eccentric than the LCA ostium23. Additionally, in this study, the angle from the native RLCC to the RCA ostium had a greater variation than the angle to the LCA ostium, which may also have contributed to the lower percentage of cases where access to the RCA was maintained. However, it was still possible to obtain a favourable orientation for coronary access in 83.3% of participants in this study, and this percentage is relatively acceptable. To increase the success rate of access to the RCA, devices need to evolve so that the orientation of the C-tab commissure more accurately matches that of the native RLCC commissure.
Limitations
This study has some limitations. First, this was a single-centre, retrospective, observational study, and the possibility of patient selection bias is undeniable. However, consecutive patients were enrolled with the same criteria for both groups, and the results of the non-#rotation group were comparable to previous studies. Second, the orientation of the delivery catheter in the #rotation method was not adjusted in the aortic valve position but in the descending aorta. Therefore, there was some deviation from the ideal orientation at the position of the aortic valve. Moreover, the position of the hat marker might deviate from the centre front at the aortic valve position due to too much torque during the passage through the aortic arch. Future improvements in devices may allow us to safely change the orientation of the THV at the level of the aortic valve. Third, we evaluated the ease of coronary access from the direction of the implanted prosthetic valve but did not attempt to engage the catheter in the coronary arteries. The ease of coronary access is also related to anatomical factors, such as the width of the sinus of Valsalva, length and calcification of the valve leaflet, and other device-related factors (such as the frame of the prosthetic valve).
Conclusions
The #rotation method allowed the C-tab commissure of Evolut to be positioned near the native RLCC commissure. This significantly improved the probability of achieving a favourable THV orientation for postoperative coronary access, especially for the LCA.
Impact on daily practice
Close alignment between the transcatheter heart valve (THV) neo-commissure and native commissure reduces the risk of overlap between the coronary artery and the THV neo-commissure. The hat marker of the delivery catheter and the C-tab of the THV are in a fixed position, and the C-tab coincides with the THV neo-commissure. By adjusting the orientation of the hat marker on the fluoroscopic display, it may be possible to implant the Evolut so that the C-tab commissure is adjacent to the native commissure.
Acknowledgements
The authors thank Dr Eiji Taguchi, Dr Toshiharu Sassa, Dr Ichiro Ideta, Dr Tomoko Nakayama, Dr Masahiro Yamada and Dr Youko Horibata for useful discussions; the Heart Team of the Saiseikai Kumamoto Hospital Cardiovascular Center for years of collaboration and advice; and Ms Naoko Takahashi (Saiseikai Kumamoto Hospital) for her statistical assistance.
Conflict of interest statement
T. Sakamoto is a clinical proctor of the Evolut TAVR system. The other authors have no conflicts of interest to declare.

