Introduction
The pharmacoinvasive strategy, defined as fibrinolysis followed by routine early percutaneous coronary intervention (PCI), is an alternative treatment strategy for patients with ST-elevation myocardial infarction (STEMI) who are unable to access primary PCI within 120 minutes1. Trials have shown that patients failing fibrinolysis should undergo immediate angiography and rescue PCI23, while those with successful fibrinolysis should undergo deferred PCI 2-24 hours following fibrinolysis to avoid unnecessary bleeding risk1. Current criteria to determine fibrinolysis success include the combination of symptom resolution, ≥50% ST-segment resolution, and the occurrence of reperfusion arrhythmias1. These criteria were validated by early streptokinase studies4, but have not been evaluated in the contemporary pharmacoinvasive era utilising modern pharmacotherapy1. This study sought to investigate the accuracy of reperfusion criteria to predict ongoing infarct-related artery (IRA) occlusion following fibrinolysis.
Methods
A single-centre retrospective analysis of all patients treated with fibrinolysis for STEMI between 2010 and 2020 was performed. Ethical approval was granted for this study. Patients were excluded from the analysis if they did not receive guideline-directed pharmacotherapy (parenteral anticoagulation and dual antiplatelet therapy) or had incomplete medical records. Demographic data, clinical characteristics on presentation, and coronary angiography results were obtained from medical records. As local practice employed prehospital fibrinolysis by emergency medical staff, data surrounding the occurrence of reperfusion arrhythmias during transport en route to the PCI centre could not be collected. Hence for this analysis, successful clinical reperfusion (SCR) was defined as symptom resolution and ≥50% reduction in ST-elevation at time of arrival at the PCI centre. The primary endpoint was IRA occlusion, defined as Thrombolysis in Myocardial Infarction (TIMI) 0-1 flow at the time of angiography following fibrinolysis. The IRA and corresponding TIMI flow for each patient were determined by a blinded interventional cardiologist. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated individually for ongoing symptoms and failure of ST-segment resolution following fibrinolysis, as well as for both criteria in combination. Data were analysed using Stata (StataCorp), and statistical significance was assumed for a p-value <0.05.
Results
A total of 495 consecutive patients with STEMI treated with fibrinolysis were identified from the hospital’s database. Of those, 53 patients were excluded as they did not receive guideline-directed pharmacotherapy, and 43 patients had incomplete medical records, thereby yielding a final cohort of 399 patients. All patients received tenecteplase and underwent immediate or deferred angiography depending on the reperfusion criteria. A total of 105 patients (26.3%) had IRA occlusion at the time of angiography. There were no differences in cardiovascular risk factors, time from symptom onset to fibrinolysis, STEMI territory, or clinical factors at presentation between patients with and without IRA occlusion.
A total of 174 patients (43.6%) had SCR, of whom 16 patients (9.2%) had IRA occlusion. Patients with SCR had a longer median time from fibrinolysis to angiography compared to those who did not achieve SCR (22.0 hours [interquartile range {IQR} 32 hours] vs 3.5 hours [IQR 4.3 hours]; p<0.01). The patients who had SCR despite having an occluded IRA had a delayed median time from fibrinolysis to angiography of 13.8 hours (IQR 22 hours). The sensitivity, specificity, PPV, and NPV for IRA occlusion were 52.4%, 70.1%, 38.5%, and 80.5%, respectively, for ongoing symptoms; 77.1%, 68.4%, 46.6%, and 89.3%, respectively, for <50% reduction in ST-elevation; and 84.7%, 53.7%, 39.6%, and 90.8%, respectively, for both criteria combined (Figure 1). There were no differences in death, heart failure, or major bleeding between groups at the time of discharge (p=0.29).
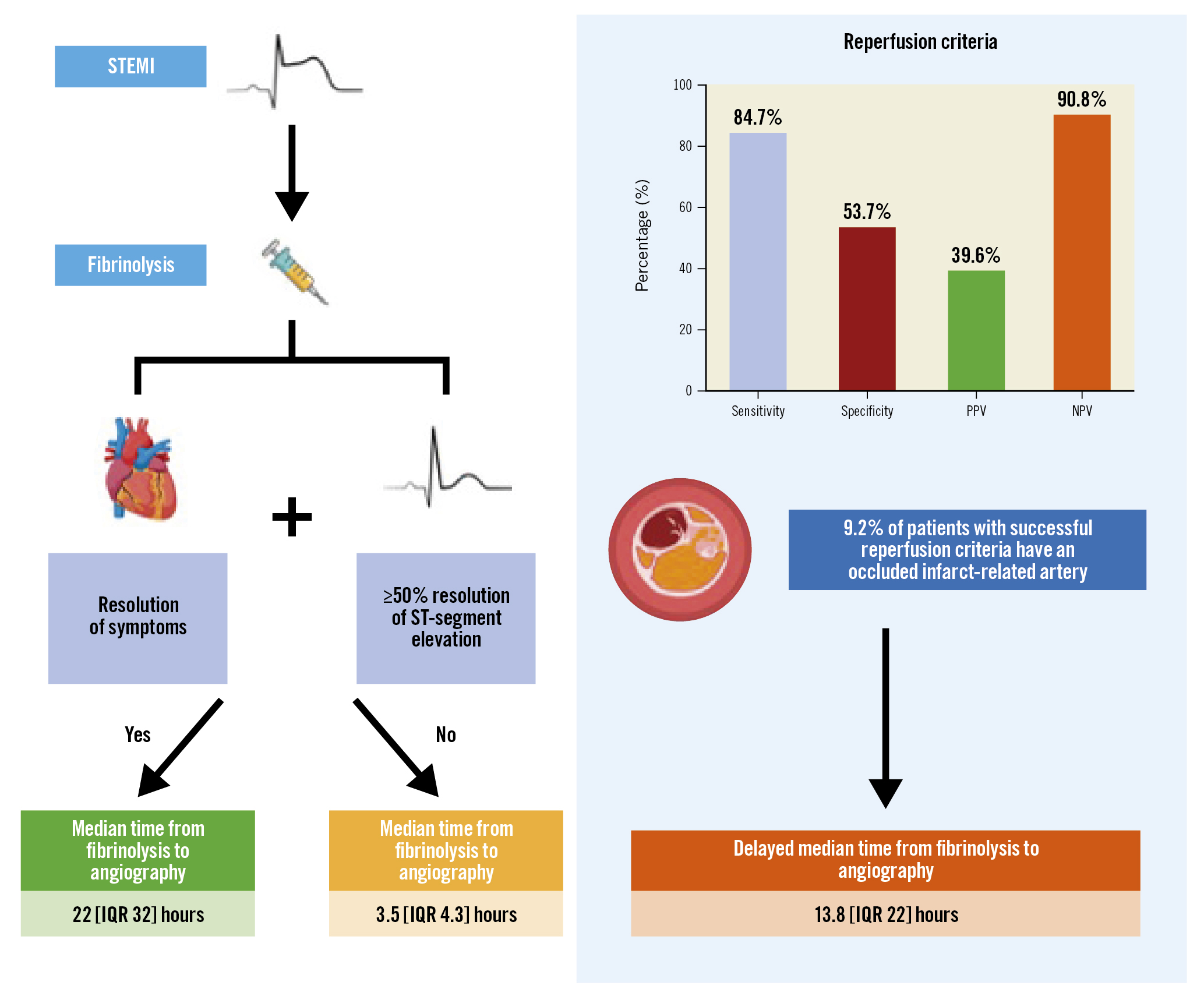
Figure 1. Diagnostic accuracy of reperfusion criteria following fibrinolysis for ST-elevation myocardial infarction. Successful fibrinolysis is determined by the resolution of symptoms and ≥50% resolution of ST-segment elevation. These criteria have modest diagnostic accuracy and may not identify occluded infarct-related arteries in up to 9.2% of patients. IQR: interquartile range; NPV: negative predictive value; PPV: positive predictive value; STEMI: ST-elevation myocardial infarction
Discussion
This study highlights that 9.2% of patients with SCR had IRA occlusion with markedly delayed time to angiography and exposure to potentially avoidable adverse ischaemic outcomes. These results are similar to those reported by early streptokinase studies4, suggesting that the accuracy of current reperfusion criteria has not improved with modern pharmacotherapy use. In an era of improved access to rescue PCI, deliberate and unnecessary delay of angiography under the false, premature assumption of SCR seems less appropriate, especially when a favourable shift in net clinical benefit is also likely to have been observed through the increasing use of radial access. Thus, a pivot towards increased sensitivity towards SCR seems prudent, with the possible incorporation of newer electrocardiogram criteria5.
Limitations
Our results need to be interpreted in the context of several limitations. Firstly, the additive, predictive utility of reperfusion arrhythmias could not be determined, although the applicability of this is often challenging due to prehospital fibrinolysis en route to PCI centres in real-world settings. Additionally, long-term clinical outcomes were not assessed due to geographical constraints with regard to follow-up, although IRA occlusion was judged to reflect poorer long-term outcomes.
Conclusions
In conclusion, the current criteria for reperfusion following fibrinolysis have a modest predictive utility for IRA occlusion and could lead to avoidable cardiovascular morbidity and mortality. In contemporary practice, where access to angiography has expanded and iterative clinical practice may have shifted the bleeding-ischaemic balance in favour of early angiography, the role of clinical reperfusion criteria may need to be revisited or at least refined towards higher sensitivity.
Funding
S. Tan is supported by a Postgraduate Scholarship from the National Health and Medical Research Council of Australia, a PhD Scholarship from the National Heart Foundation of Australia, and an Australian Government Research Training Program Scholarship. A. Nelson is supported by a Postdoctoral Fellowship from the National Heart Foundation of Australia. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
S. Nicholls received research support from AstraZeneca, New Amsterdam Pharma, Amgen, Eli Lilly, Anthera, Novartis, Cerenis, The Medicines Company, Resverlogix, Sanofi-Regeneron, InfraReDx, Esperion, Roche and Liposcience; consulting fees/honoraria from AstraZeneca, Amarin, Akcea, Eli Lilly, Anthera, Omthera, Merck, Takeda, Resverlogix, Sanofi-Regeneron, CSL Behring, Esperion, and Boehringer Ingelheim. The other authors have no conflicts of interest to declare.
