Introduction
Percutaneous treatment of heavily calcified coronary lesions remains a common challenge which is present in one-third of patients suffering from stable and acute coronary syndromes, and portends worse procedure-related and patient-related outcomes12. Severity of coronary artery calcification increases with age, presence of cardiovascular risk factors and comorbid conditions34. Percutaneous coronary intervention (PCI) in calcified lesions has been associated with major adverse cardiac events (MACE), increased rates of stent thrombosis, restenosis, and target lesion revascularisation12.
Coronary calcification also increases procedural time and complexity. Moreover, the extent of coronary calcification (depth, arc, length and thickness) influences procedural success, stent delivery and expansion, and also potentially impairs drug delivery as well as damaging stent polymer56. Several technologies have been developed over the last three decades to overcome this hurdle, including cutting/scoring balloons, high-pressure non-compliant balloons, rotational or orbital atherectomy, and excimer laser789. These modalities rely upon tissue debulking or tissue compression and are associated with increased procedural complications such as coronary intimal layer dissection, coronary perforation, and distal embolisation710. So far, atherectomy devices and speciality balloons have not been proven to be superior to non-compliant balloons in improving clinical outcomes8911.
Intravascular lithotripsy (IVL) is a new addition to the armamentarium for treatment of calcified coronary lesions12. The IVL system transforms electrical energy into sonic waves that selectively fracture superficial and deep calcium at low-pressure balloon dilatation thus minimising vessel wall barotrauma1213. The safety and efficacy of IVL were demonstrated in a series of DISRUPT CAD clinical trials121314. In this study we present early single-centre Australian experience with the use of IVL in the treatment of severe coronary calcification in real-world patients treated for chronic and acute coronary syndromes.
This manuscript was approved by the Fiona Stanley Hospital Ethics Committee.
Methods
Study setting
We performed a retrospective analysis of all patients who underwent PCI and IVL shockwave therapy at our quaternary hospital from October 2019 to June 2021. Patient demographics and procedural data were prospectively collected and analysed. Indication for PCI was either stable coronary artery disease (CAD) with evidence of ischaemia or acute coronary syndromes (ACS), or single- or multivessel CAD with a diameter stenosis ≥70%. IVL was used in severely calcified coronary lesions resistant to high-pressure non-compliant balloon inflation (>18 atm) or upfront as an alternative to, or in combination with, traditional calcium modifying devices such as rotational atherectomy or ultrahigh-pressure balloons. Another indication for IVL therapy was bailout treatment of significantly underexpanded stents (>30% stenosis). There were no exclusion criteria.
Study device
The intravascular lithotripsy catheter (IVL; Shockwave Medical Inc.) is a monorail rapid exchange catheter that delivers an integrated semi-compliant balloon containing two lithotripsy emitters. The IVL balloon is filled with diluted contrast, with the recommended therapy delivery inflation pressure of 4 atmospheres (atm), a nominal pressure of 6 atm and a rated burst pressure of 10 atm. Sonic waves delivered at 4 atm balloon inflation generate peak pressure of approximately 50 atm15. Available IVL catheters are from 2.5 mm to 4.0 mm in diameter and 12 mm in length1213. The IVL catheter is connected to the generator via a cable and is preset to deliver 10 sonic wave pulses per cycle (one pulse/second) for a maximum of eight cycles. The IVL balloon is sized 1:1 to the reference artery and inflated at a minimum of 4 atm to deliver acoustic energy and maximum of 10 atm if required to assess symmetrical expansion and confirm calcium modification.
Procedures
PCIs were performed by experienced operators, and national guidelines were followed for assessment and treatment of acute and chronic coronary syndromes. The decision to proceed with IVL therapy was based on the discretion of the individual interventional cardiologist once they had concluded that high-pressure (>18 atm) non-compliant balloon inflation was ineffective to dilate the lesion adequately. Operators proceeded with any adjunctive tool to perform optimal PCI; these could be scoring/cutting balloons, or rotational atherectomy (note: orbital atherectomy is currently not available in Australia). Intravascular imaging was used as required, but its use was not universal. Stent implantation with a drug-eluting stent (DES) and PCI optimisation were performed as per standard of care.
Endpoints and definitions
The primary safety endpoint was defined as freedom from MACE within 30 days following the index procedure. MACE was defined as cardiac death, myocardial infarction (MI), target lesion revascularisation, or stroke. Angiographical success was defined as success in stent delivery and <20% residual diameter stenosis after stenting of the target lesion, assessed by quantitative coronary angiography (QCA). Clinical success was defined as success in stent delivery and <20% residual diameter stenosis after stenting of the target vessel with no MACE.
Stent thrombosis was defined as definite stent thrombosis by angiography, and procedural myocardial infarction (MI) was defined according to the universal definition of MI16. This includes a troponin rise >x5 upper level of normal as well as one of the following: ischaemic electrocardiogram changes, new Q-waves, angiographic complications such as severe dissection, side branch occlusion or slow flow. Severe angiographic calcification was defined as radio-opacities observed without cardiac motion, usually affecting both sides of the arterial lumen, mimicking a double track line or guided by intracoronary imaging. Serious coronary intervention complications were recorded and defined as severe coronary dissection, coronary perforation, slow-flow or no-reflow phenomenon, and abrupt vessel closure.
Statistical analysis
Continuous variables are described as mean±standard deviation (SD). Categorical variables are described as proportions.
Results
Patients and procedures
Between October 2019 and June 2021, 40 patients and 41 coronary lesions were treated with the coronary IVL system in our institution. Patients’ baseline and coronary lesion characteristics are provided in Table 1 and Table 2, respectively. Fifteen patients (37.5%) underwent IVL-assisted PCI for treatment of stable CAD with evidence of ischaemia, and 25 patients (62.5%) had IVL therapy during invasive treatment of ACS (ST-segment elevation myocardial infarction [STEMI] 17.5%, non-ST-segment acute coronary syndrome [NSTEACS] 45%).
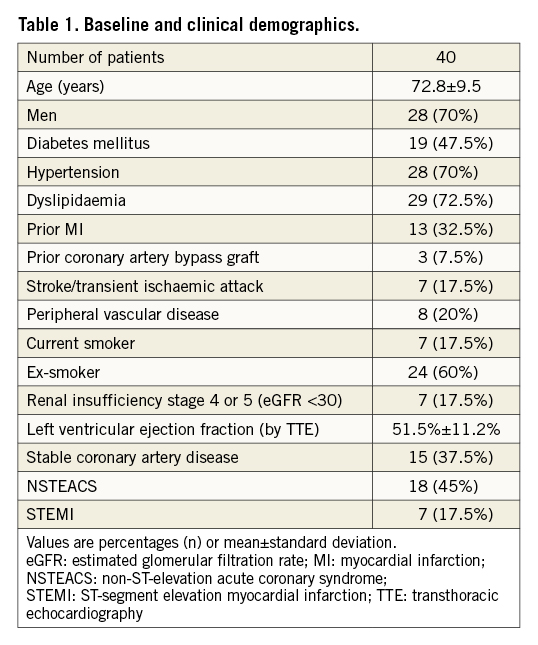
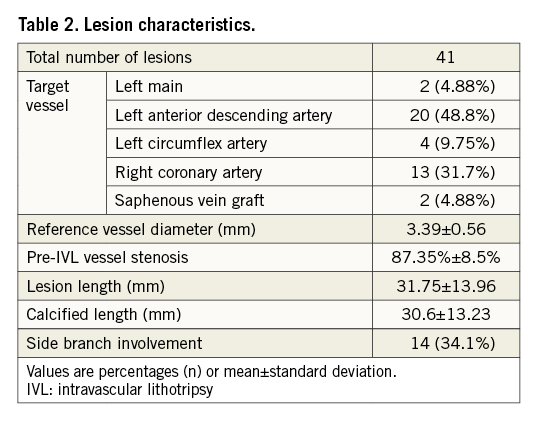
The left anterior descending artery was the most common target vessel (48.8%). The mean reference vessel diameter was 3.38±0.61 mm with a mean lesion length of 32.4±18.4 mm. Calcification was extensive with a mean calcified length by coronary angiography of 33.7±12.2 mm.
Procedural details are provided in Table 3. Mean overall procedure duration was 88.17±38.9 minutes. However, 11 patients (27.5%) underwent two-vessel PCI including one chronic total occlusion PCI. Excluding these cases, the mean procedural time would be reduced to 77.7 minutes. One IVL catheter was used per patient in all cases. Predilatation prior to IVL use was performed in 38 (95%) cases. Upfront atherectomy with a 1.5 mm burr was required in one case as the IVL catheter was unable to cross the lesion, and successful predilatation with a 2.5 mm OPN ultrahigh-pressure balloon (SIS Medical) was used in one patient after IVL crossing failure. IVL treatment was provided in these two cases to maximise calcium modification.
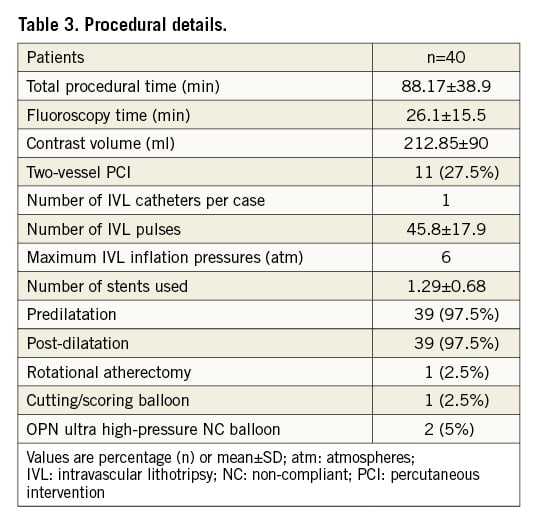
An average number of 1.29±0.68 DES were implanted per subject in 39 patients. One patient had drug-coated balloon angioplasty (SeQuent; B. Braun) of severe calcific stenosis inside two layers of underexpanded stents. Post-dilatation following stent implantations was performed in 39 (97.5%) patients. In four cases, IVL was used after new stent implantation due to >30% residual diameter stenosis and suboptimal stent expansion. Three patients underwent IVL therapy for treatment of severe calcification and stenosis at the site of old underexpanded stents.
Endpoints
The in-hospital primary safety endpoint occurred in two patients, consisting of two MIs, neither of which were directly related to IVL treatment delivery. One case developed MI as a result of coronary dissection in PCI of a non-calcified lesion where a semi-compliant balloon was used for predilatation. The second patient with MI was in a case of coronary perforation managed by a covered stent sacrificing a diagonal branch. Two patients passed away in the follow-up period because of non-cardiac causes.
Clinical and angiographic success with <20% residual stenosis was achieved in 90% and 92.5% of patients, respectively. IVL catheter delivery, treatment of the target lesion, and subsequent stent delivery were successful in all patients. In four cases, the IVL catheter failed to cross the coronary lesion. Initial IVL therapy was delivered with only the distal balloon marker engaging the proximal lesion edge, which probably partially modified the calcified lesion and facilitated subsequent IVL balloon advancement through the stenosis.
The mean final residual stenosis post-IVL and stent implantation was 8.25%±8.5% (Table 4). One coronary perforation (the same individual with MI due to sacrificed diagonal branch) occurred after post-dilatation of the implanted stent with a non-compliant balloon which was managed with a covered stent implantation (PK Papyrus; Biotronik)17. There was one case of IVL therapy-induced coronary dissection, limited to the treated coronary segment, which was successfully covered with a stent (Table 5). Representative angiographic and intracoronary imaging examples of the effects of IVL are shown in Figure 1-Figure 3.
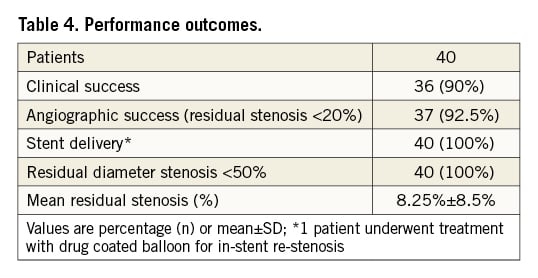
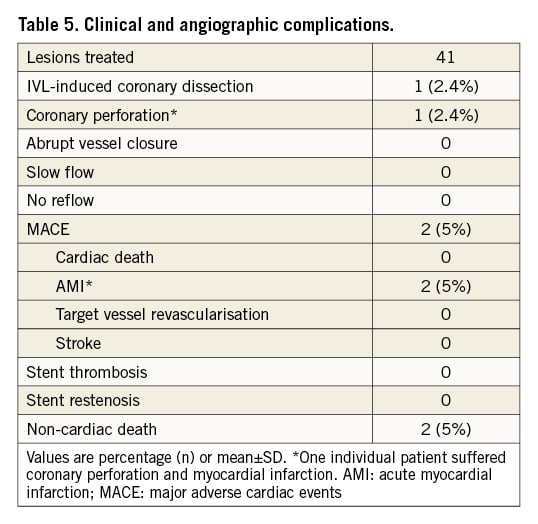
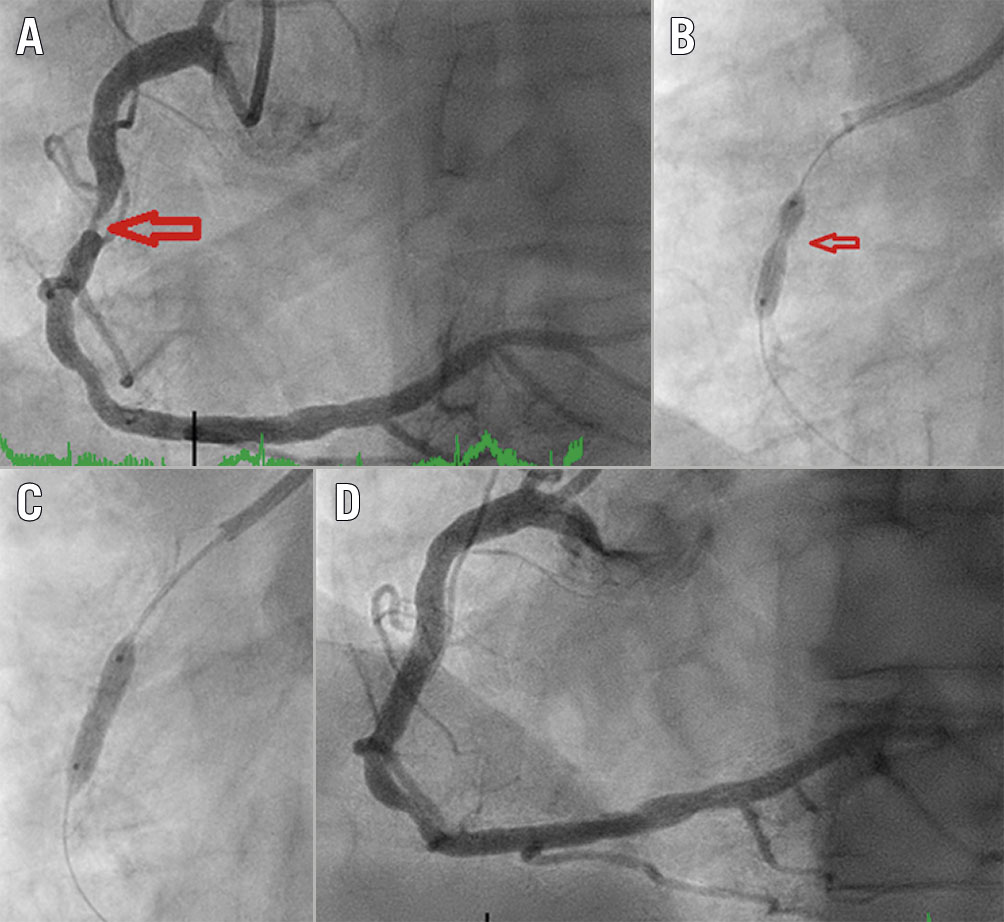
Figure 1. Fluoroscopic demonstration of IVL-assisted angioplasty of a calcified right coronary artery stenosis. A) Diagnostic angiogram of right coronary artery with severe stenosis. B) NC balloon inflation (3.5/12 mm inflation at 20 atm) showing undilatable segment (arrow). C) Full expansion of IVL 3.5/12 mm balloon at 4 atm and one therapy cycle provided. D) Coronary angiography following stent implantation. IVL: intravascular lithotripsy; NC: non-compliant
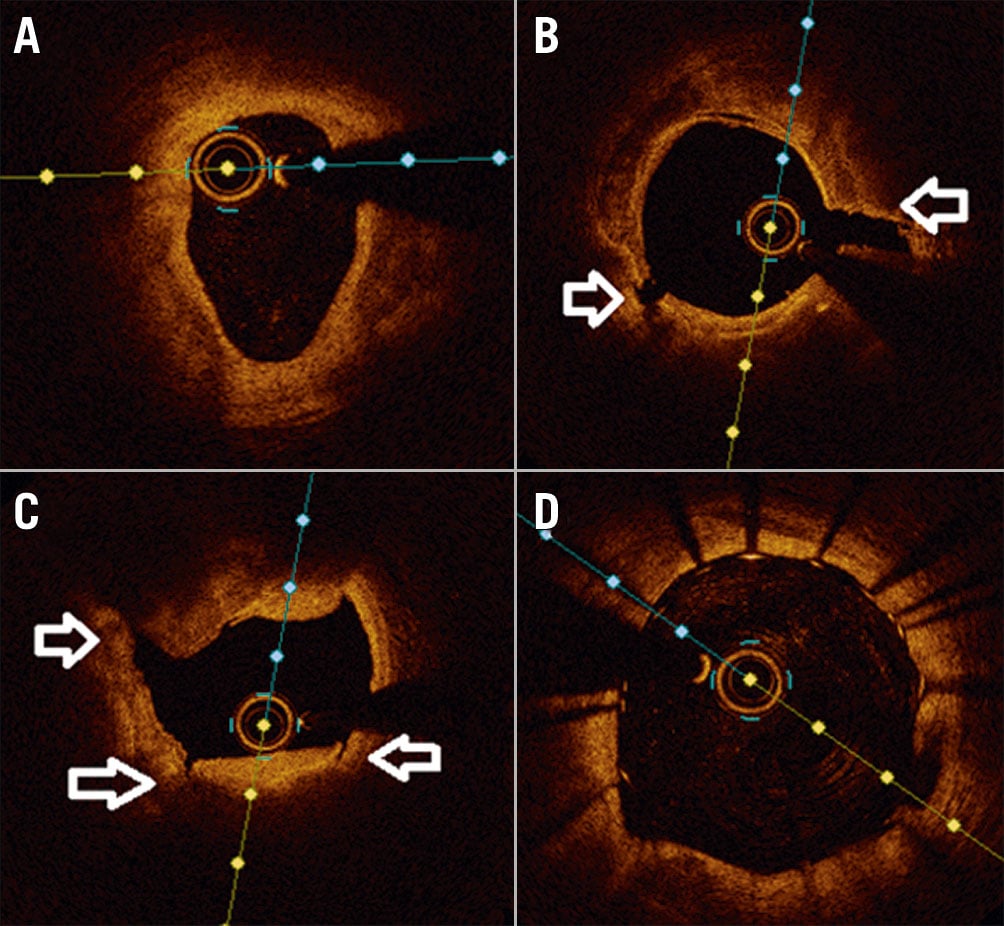
Figure 2. Intracoronary imaging demonstration of IVL-assisted angioplasty of a severely calcified coronary stenosis. A) Pre-lithotripsy optical coherence tomography (OCT) scan showing severe calcification, B), C) Post-lithotripsy OCT showing fractured calcium (arrows), D) Final OCT image of stented vessel demonstrating well expanded/apposed stent.
Figure 3. Use of IVL in an underexpanded stent. A) Diagnostic angiogram of right coronary artery showing significant stent underexpansion. The stent (arrow) was inserted at another institution 48 hours prior to this admission. B) OCT image showing severe coronary calcification. C) NC balloon inflation (4.0/15 mm inflation at 20 atm) showing undilatable stent . D) Full expansion of IVL 4.0/12 mm balloon at 6 atm. E) OCT post-IVL Shockwave therapy showing calcium fractures and expanded stent. F) Post-IVL angiography showing well-expanded stent with residual 10% stenosis. IVL: intravascular lithotripsy; NC: non-compliant; OCT: optical coherence tomography
Discussion and limitations
Our retrospective study demonstrated the feasibility and safety of IVL therapy in preparation of severely calcified coronary stenoses prior to stent implantation in a real-world population. We also safely performed IVL therapy within three newly inserted stents with significant underexpansion of >30%, and in two patients with severe calcific stenoses at the sites of old stents. Major clinical and procedural findings of our study include: 1) coronary IVL therapy is safe and beneficial in treatment of severe coronary calcification; 2) the procedure was well tolerated with 100% success in stent delivery and a low risk of periprocedural complications; 3) IVL therapy can be combined with other adjunctive therapies for balloon non-dilatable/non-crossable calcific lesions in order to achieve optimal procedural outcomes; 4) the IVL device is easy to use with less technical complexity and preparation than rotational atherectomy.
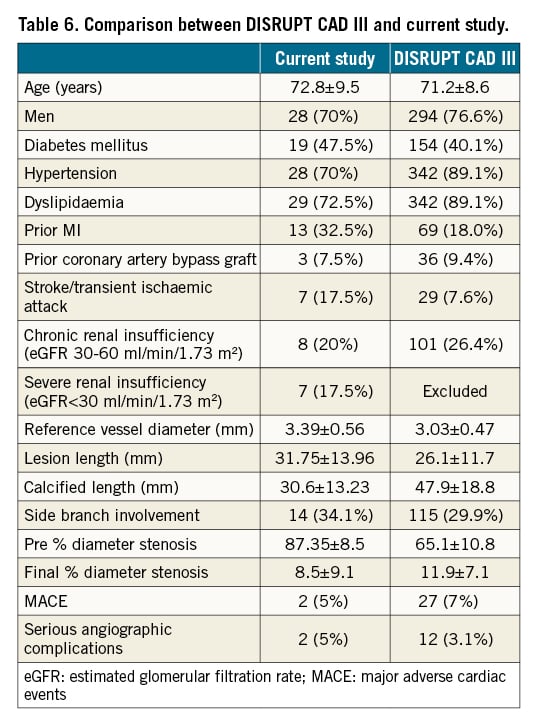
Our findings are comparable to the recently published DISRUPT CAD III14 study which was designed to assess the safety and effectiveness of IVL as an adjunct therapy in calcific coronary PCI. DISRUPT CAD III is a non-randomised single-arm multicentre prospective study which achieved the prespecified performance goals, prompting US regulatory approval in the utilisation of this technology for treatment of calcific coronary artery disease. However, there are some differences between our study and DISRUPT CAD III. First, in the DISRUPT CAD III, only de novo coronary lesions were included, whereas we also reported the performance of IVL in the treatment of calcified stenoses in coronary arteries stented years ago, and in newly implanted stents with >30% residual stenosis due to underexpansion in calcified lesions. While not currently supported by the product developer, off-label use of IVL to treat in-stent restenosis due to calcific neoatherosclerosis18 and to expand old underexpanded stents19 has been reported. Currently, no evidence exists to support the safety of the immediate use of IVL in underexpanded stents, and concerns regarding possible disruption to the stent architecture and/or drug/polymer coating have been raised. However, we believe that rectifying in-stent restenosis or expanding an underexpanded stent holds a distinct clinical benefit to the patient, reducing the exceedingly high risk of stent thrombosis/restenosis5202122 and subsequently MACE. The safety and efficacy of IVL therapy in newly inserted stents was similar to IVL in the treatment of de novo coronary lesions, although long-term follow-up data is lacking. We recommend appropriate lesion preparation and calcium modification before stent insertion, until further studies demonstrate the safety of this novel technology in newly inserted stents as a bailout option. The risks and benefits must be taken into account and this should only be considered on the rare occasions operators encounter suboptimal stent results.
Second, the majority of patients (95%) included in this study underwent IVL as a bailout following failed standard balloon angioplasty, whereas predilatation prior to IVL was performed in 55% of DISRUPT CAD III lesions. Intracoronary imaging was used infrequently in both studies (25% in DISRUPT CAD III and 30% in this study). More routine use of intracoronary imaging would be beneficial to identify the lesions with moderate to severe calcification which would benefit from upfront calcium modification treatment.
Third, DISRUPT CAD III excluded patients with ACS, severe chronic kidney disease, saphenous vein grafts (SVG) and use of atherectomy or cutting/scoring balloons, which restricted the use of the IVL system to a subgroup of select patients. Our study included all patients who we believed would benefit from this novel technology including those with multivessel coronary disease, chronic total occlusions, in-stent restenosis, and underexpanded de novo stents, reflecting a real-world and diverse population. Our limited experience with the use of IVL in ACS patients or SVG PCI did not demonstrate any additional adverse outcomes, such as arrythmias due to “shocktopics” or slow-flow/no-reflow phenomena.
With regard to efficacy endpoints, DISRUPT CAD III defined procedural success as stent delivery with residual stenosis <50%, which is considered unsatisfactory and a suboptimal outcome in coronary intervention. The recommended stent expansion is at least 80% and ideally >90% with <20% residual stenosis23. Therefore, we defined angiographic success as <20% residual stenosis. Considering safety endpoints, we observed a similar serious angiographic complication rate with IVL therapy compared to the DISRUPT CAD III study (5% vs 7%, respectively). Table 6 compares the findings between the DISRUPT CAD III and the current study.
Finally, one of the major setbacks in IVL is lesion crossing failure. Investigators in the DISRUPT CAD trial were not allowed to use other adjunctive coronary lesion preparation devices as part of the study protocol. This resulted in 8 IVL catheter crossing failures, however, we opted to combine IVL with other therapies in this group of patients. These technologies are complementary with different modes of action, and use of one does not preclude the use of another. Rotational and orbital atherectomy were both shown to improve procedural outcomes in ROTAXUS8 and ORBIT II9 trials, respectively. These modalities facilitate stent delivery and implantation by debulking calcified coronary stenoses, whereas IVL creates microfractures in coronary calcification, facilitating better stent expansion. As opposed to atherectomy debulking techniques, IVL therapy may possibly reduce the likelihood of atheromatous distal embolisation and associated risk of slow-flow/no-reflow phenomena12. Atherectomy devices primarily modify superficial coronary calcification, whereas IVL fractures both deep and superficial calcium layers2425. Whether the combination of atherectomy and IVL improves outcome over either modality alone remains unclear and requires further study.
High-pressure non-compliant balloons and ultrahigh-pressure balloons (OPN; SIS Medical) are therapies that have been used over decades, however there is no compelling evidence to support improved procedural and/or clinical outcomes with their use. On the other hand, preclinical data suggest the resultant excessive coronary vessel wall stretch and deep injury are potent stimuli for neointimal hyperplasia2627. Currently, little evidence is available on the impact of IVL therapy on neointimal hyperplasia. It is, however, postulated that due to a low balloon inflation pressure of 4 atm, there would be less barotrauma to the vessel wall and potentially less neointimal hyperplasia. Also, IVL differentially fractures hard calcium layers rather than affecting soft intimal and medial tissues, potentially reducing vessel wall injury13.
These findings are representative of a single Australian centre and may not be generalisable to other institutions. This is an observational retrospective study lacking a concurrent control group, and not all variables are considered in the outcomes. Infrequent use of intracoronary imaging to ensure optimal lesion preparation and stent implantation is a disadvantage in our cohort. There is no long-term follow-up for our patients and future studies are required to investigate long-term procedural and clinical outcomes after IVL therapy.
Conclusions
In conclusion, our findings confirm the short-term safety and efficacy of IVL to manage severely calcified coronary lesions in broader clinical scenarios from stable CAD to ACS. Our outcomes are aligned with the current limited clinical trials, such as DISRUPT CAD III. Combination of the IVL Shockwave system with other adjunctive therapies is a viable option in balloon non-dilatable/uncrossable calcific stenosis.
Impact on daily practice
Our results demonstrate real-world short-term safety and efficacy of IVL to manage severely calcified coronary lesions in acute and chronic coronary syndromes. Combination of the IVL Shockwave system with other adjunctive therapies is a viable option in calcific stenoses which are undilatable/uncrossable by balloon. The IVL Shockwave system is easy to use, with less technical complexity and preparation than rotational atherectomy.
Acknowledgements
The authors would like to thank the entire cardiology department staff involved in the care of patients at Fiona Stanley Hospital.
Conflict of interest statement
The authors have no conflicts of interest to declare.

