When performing transcatheter aortic valve replacement (TAVR), intraprocedural rapid ventricular pacing is mandatory to ensure transient ventricular standstill during certain moments of transcatheter aortic valve (TAV) positioning and deployment, as well as during pre- and post-balloon dilatation, if necessary. Compared with right ventricular (RV) stimulation using a temporary pacing lead, left ventricular (LV) stimulation during TAVR has been shown to be associated with significantly reduced procedure duration, fluoroscopy time, and cost, with similar efficacy and safety1. The conventional LV pacing-over-the-wire technique utilises an external pacemaker (pulse generator) with two alligator clamps. One alligator clip, which acts as a cathode, is attached to the external part of the LV guidewire, with either the balloon catheter or TAV delivery catheter as an insulator. Another alligator clip, which acts as a grounding anode, is attached to the patient’s subcutaneous tissue by either a fine-gauge needle or directly biting the skin at the edge of the skin incision1. The major disadvantages of this conventional LV pacing technique include (1) the patient’s discomfort, incurred when attaching the alligator clip to the subcutaneous tissue, (2) inadvertent muscle stimulation at the groin region where the alligator clip is attached to the subcutaneous tissue, and (3) risk of operator injury if a fine-gauge needle is used to attach the alligator clip. Therefore, a novel LV pacing-over-the-wire technique, the SENTIPACE technique for TAVR using the SENTINEL (Boston Scientific) cerebral protection device (SENTIPACE: SENTInel coronary guidewire – LV guidewire PACing stratEgy), was invented to mitigate the above-mentioned disadvantages and further streamline TAVR procedural steps2.
The current study was a physician-initiated, prospective, single-arm, first-in-human, pilot study using the SENTIPACE technique for intraprocedural rapid ventricular pacing during TAVR. The study included 25 consecutive patients who underwent transfemoral TAVR with the SENTINEL cerebral protection device at Queen Elizabeth Hospital, Hong Kong SAR, during 2023. It was approved by an internal ethical committee. All patients provided written informed consent. In the SENTIPACE technique, the alligator clip that acts as the grounding anode is attached to the external part of the coronary guidewire that is loaded in the SENTINEL device, instead of the patient’s subcutaneous tissue. The other alligator clip, which acts as a cathode, is attached to the external part of the LV guidewire (Figure 1). The ventricular output of the pacemaker delivering a constant voltage (V) was standardised and set at an output of 10 V. The primary endpoint was the efficacy and safety of pacemaker stimulation. Efficacy was defined as achievement and maintenance of a systolic blood pressure of less than 60 mmHg for more than 10 seconds without loss of capture. The safety of pacemaker stimulation was characterised by a combination of procedural success and major adverse clinical and cerebrovascular events.
A total of 25 patients were enrolled. Baseline patient, procedural and postprocedural characteristics are reported in Table 1. Effective ventricular stimulation was achieved in all patients using a voltage output at 10 V. There was no conversion to conventional LV pacing-over-the-wire or RV stimulation strategy. Both balloon-expandable valves (SAPIEN 3 [Edwards Lifesciences]; 20%) and self-expanding valves (Evolut PRO+ [Medtronic], Navitor [Abbott], ACURATE neo2 [Boston Scientific], ALLEGRA [Biosensors]; 80%) were used. The TAVR procedural success rate was 100% with a single TAV deployed. There was no more than or equal to moderate paravalvular leakage on the transthoracic echocardiogram post-TAVR. There were no cerebrovascular events at clinical follow-up at 30 days. One patient, who had severe aortic valve stenosis with septic shock and emergency TAVR, succumbed at day 14 post-TAVR because of severe pneumonia. In one patient, TAVR was complicated with a ruptured sinus of Valsalva and cardiac tamponade, though the procedure was successfully rescued by pericardiocentesis and coil embolisation.
The main finding of this pilot study was that use of the LV stiff wire and coronary guidewire in the SENTINEL device for rapid ventricular pacing during TAVR was effective and safe across different TAV platforms. LV stimulation was shown to be as effective as RV stimulation and to have advantages over a standard temporary pacing wire1. By further modifying the LV stimulation technique, the SENTIPACE pacing strategy allows further simplification and streamlining of the TAVR procedure.
The current first-in-human study lacks the power needed to determine the statistical significance of the main safety analysis or of the effectiveness endpoint. A larger randomised study would be needed to confirm our findings. The minimal pacing threshold was not tested. Although effective ventricular stimulation was achieved in all patients using a voltage output at 10 V in this study, a higher voltage output can be adopted in cases where effective ventricular stimulation cannot be achieved, as the pacing threshold depends on different combinations of guidewires and patient body resistance.
In this pilot study, the SENTIPACE technique was shown to provide a safe and effective method for rapid ventricular pacing during TAVR with the SENTINEL cerebral protection device. This pacing strategy can effectively and reliably streamline TAVR by further reducing procedural steps.
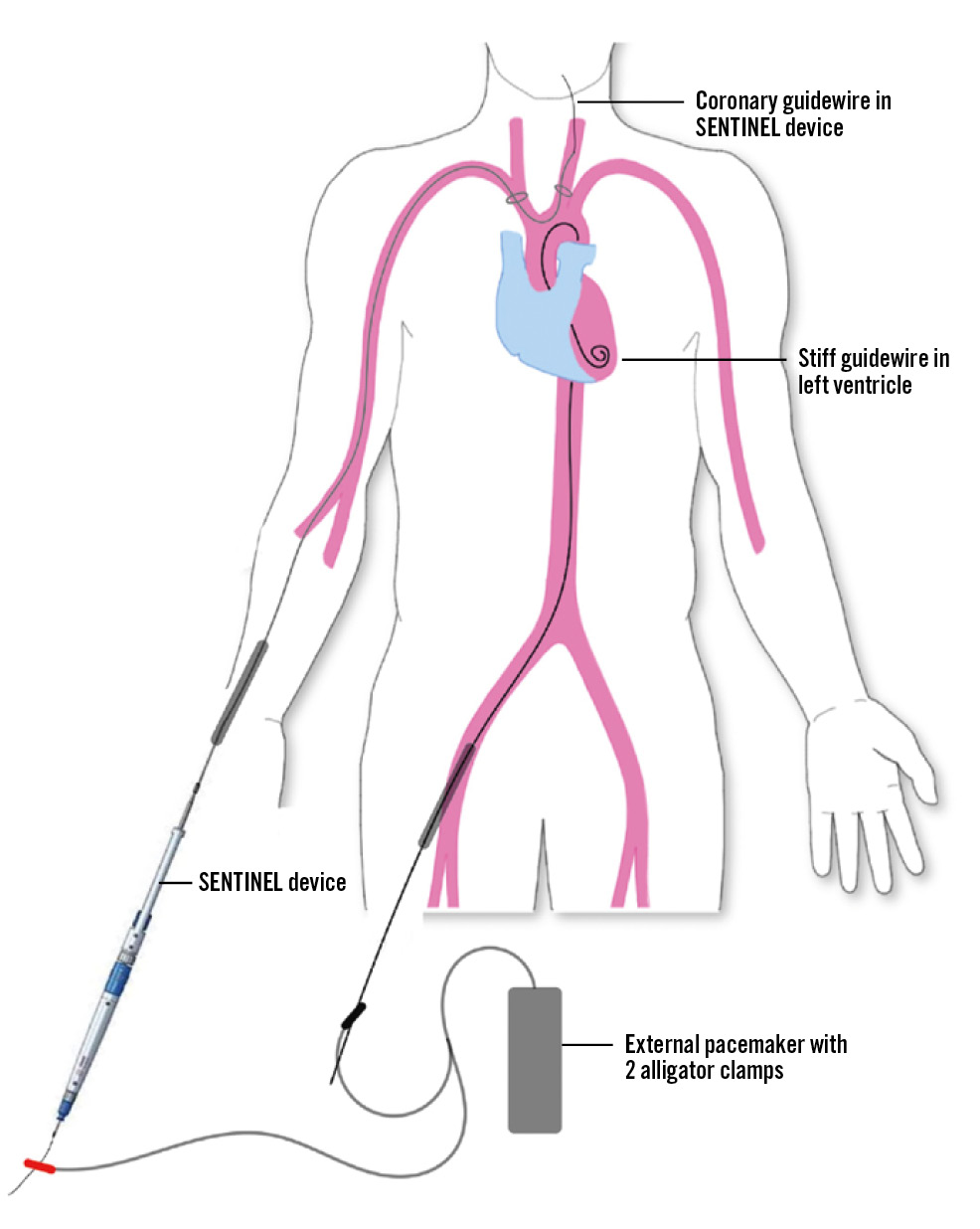
Figure 1. Schematic diagram of the SENTIPACE setup during TAVR. Either a balloon catheter or TAV delivery catheter is required as an insulator over the LV stiff wire. LV: left ventricle; TAV: transcatheter aortic valve; TAVR: transcatheter aortic valve replacement
Table 1. Baseline patient, procedural and postprocedural characteristics.
| Characteristic | Whole study population (N=25) |
|---|---|
| Age, yrs | 79.76±8.85 |
| Sex | |
| Male | 10 (40) |
| Female | 15 (60) |
| BSA, m2 | 1.62±0.18 |
| Medical history | |
| Hypertension | 14 (56) |
| Diabetes | 9 (36) |
| Hyperlipidaemia | 16 (64) |
| Smoker | 2 (8) |
| Previous MI | 0 (0) |
| Previous TIA/stroke | 2 (8) |
| Previous PCI | 6 (24) |
| Previous CABG | 0 (0) |
| Previous cardiac surgery | 1 (4) |
| Pacemaker | 3 (12) |
| STS score, % | 5.00±3.57 |
| LVEF, % | 51.10±14.49 |
| AVA, cm2 | 0.62±0.14 |
| Transaortic mean gradient, mmHg | 52.24±16.72 |
| TAV type | |
| SAPIEN 3a | 5 (20) |
| Evolut PRO+b | 11 (44) |
| Navitorc | 5 (20) |
| ACURATE neo2d | 2 (8) |
| ALLEGRAe | 2 (8) |
| Number of TAV devices implanted 1 |
25 (100) |
| Type of coronary guidewire used inside SENTINELd device | |
| BMW UNIVERSAL IIc | 22 (88) |
| Runthrough NS Floppyf | 3 (12) |
| Type of stiff guidewire used in left ventricle SAFARI2d |
25 (100) |
| Efficacy of pacing stimulation | 25 (100) |
| Predilatation | |
| Yes | 24 (96) |
| No | 1 (4) |
| Post-dilatation | |
| Yes | 10 (40) |
| No | 15 (60) |
| Procedural success | 25 (100) |
| Procedural duration, min | 81.96±48.36 |
| Fluoroscopy time, min | 26.40±17.76 |
| Postprocedural LVEF, % | 54.0±10.5 |
| Postprocedural AVA, cm2 | 1.85±0.46 |
| Postprocedural mean gradient, mmHg | 9.38±4.52 |
| Postprocedural paravalvular leak | |
| None | 15 (60) |
| Trivial | 5 (20) |
| Mild | 5 (20) |
| Clinical outcomes at 30 days | |
| All-cause mortality | 1 (4) |
| Permanent pacemaker | 1 (4) |
| Haematoma | 1 (4) |
| Cardiac tamponade | 1 (4) |
| Stroke | 0 (0) |
| MI | 0 (0) |
| Values are mean±standard deviation, or number (%). aBy Edwards Lifesciences; bby Medtronic; cby Abbott; dby Boston Scientific; eby Biosensors; fby Terumo. AVA: aortic valve area; BMW: BALANCE MIDDLEWEIGHT; BSA: body surface area; CABG: coronary artery bypass graft; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PCI: percutaneous coronary intervention; STS: Society of Thoracic Surgeons; TAV: transcatheter aortic valve; TAVR: transcatheter aortic valve replacement; TIA: transient ischaemic attack | |
Conflict of interest statement
The authors have no conflicts of interest to declare.

