Patients with acute coronary syndrome (ACS) generally have a higher risk of revascularisation after percutaneous coronary intervention (PCI) and a worse long-term prognosis than patients with stable angina1. The incidence of cardiovascular events in the PACIFIC study, a prospective multicentre ACS registry in Japan, was more than twice the incidence in all patients with coronary artery disease (>35/1,000 vs 4.5-15/1,000 per year, respectively) within 1 year after ACS onset23.
Drug-eluting stents (DES) are frequently used regardless of the presence of ACS3. Although a DES can more effectively reduce the risk of in-stent restenosis (ISR) than a bare metal stent (BMS), DES are associated with specific complications, such as delayed thrombosis after DES implantation (late or very late stent thrombosis). Neoatherosclerosis (NA), the development of new atherosclerotic lesions at the site of stent implantation4, is thought to be one of the causes of thrombosis in these cases. Optical coherence tomography (OCT) is one of the most effective imaging modalities with which to detect atherosclerotic changes within the neointima5. Researchers who observed ISR lesions using OCT reported that significantly unstable lesions (thin-cap fibroatheroma-containing neointima, neointimal rupture, and thrombi) were observed in patients with unstable angina after DES implantation5. We hypothesised that because DES implantation in unstable lesions causes early NA, patients with ACS may have a higher adverse event rate after PCI. However, most studies to date have focused on OCT observation of ISR lesions, involved a long period of time from stent implantation to in-stent evaluation, and utilised first-generation DES.
In this study, we used OCT to compare the in-stent findings at 1 year in patients with and without ACS treated with a second-generation DES.
Methods
STUDY POPULATION
In total, 703 patients who underwent PCI at our institution from March 2017 to November 2020 were retrospectively identified. Among them, 359 patients (484 lesions) were selected for this study after excluding patients without newly stented lesions. Of these, 102 patients (122 lesions) who were followed up with in-stent observation using OCT were enrolled. Patients who had undergone PCI <90 or >366 days prior to in-stent OCT observation were excluded. Patients who underwent in-stent observation using intravascular ultrasound (IVUS) were also excluded (Figure 1). If a patient had 2 or more lesions, only the first lesion treated was included in the lesion analysis. This study was approved by the Ethics Committee of Osaka Metropolitan University Graduate School of Medicine, Osaka, Japan (approval number: 2020-271) and performed in accordance with the Declaration of Helsinki.
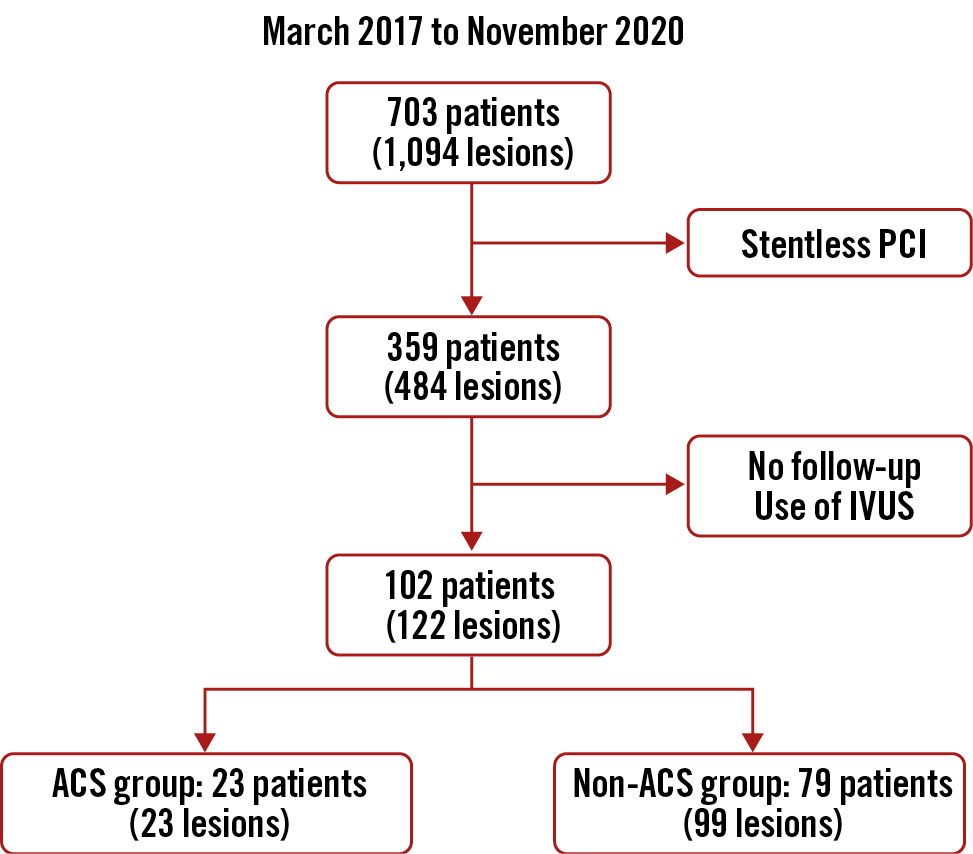
Figure 1. Study flow diagram. ACS: acute coronary syndrome; IVUS: intravascular ultrasound; PCI: percutaneous coronary intervention
INTRAVASCULAR ULTRASOUND/OPTICAL COHERENCE TOMOGRAPHY IMAGE ACQUISITION
IVUS images were acquired using an AltaView imaging catheter (Terumo) or OptiCross imaging catheter (Boston Scientific). OCT image acquisition was performed using a FastView catheter (Terumo) with the Lunawave optical frequency domain imaging system (Terumo) or a Dragonfly JP catheter (Abbott) with the ILUMIEN OPTIS imaging system (Abbott). The OCT catheter was pulled back at a rate of 20 mm/s. Contrast medium was continuously flushed through a guiding catheter at a rate of 3 mL/s for a duration of 4 to 5 seconds. Continuous images were acquired and stored digitally for analysis.
OPTICAL COHERENCE TOMOGRAPHY IMAGING ANALYSIS
The criteria for the diagnosis of NA were lesions with lipid-laden neointima, neointima with calcification, a thin-cap fibroatheroma-like neointima, or neointimal rupture. Lipid-laden neointima was defined as a signal-poor region with diffuse borders, and neointima with calcification was defined as a well-delineated, signal-poor region with sharp borders (Figure 2). The lipid arc was measured at every 1 mm interval, and the plaque length was measured. The lipid volume index was then calculated as the mean lipid arc multiplied by the lipid core length, with the length being defined as that containing >90 degrees of lipid6. The localisation and properties of NA were examined by 3 investigators (K. Nakao, T. Yamaguchi, and T. Yamazaki), and lipid NA was analysed by three investigators (N. Fujisawa, K. Otsuka, and T. Yamazaki).
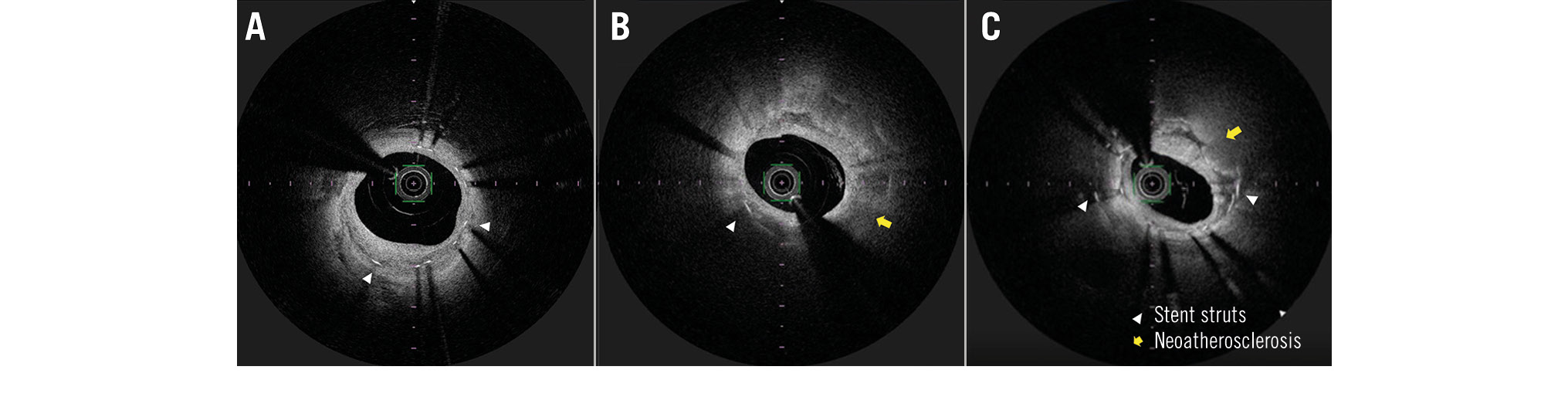
Figure 2. Representative optical coherence tomography images of neointima and neoatherosclerosis. A) Image of normal neointima. Homogeneous tissue deposits are present inside the stent struts. B) Image of lipid neoatherosclerosis. Heterogeneous tissue deposits with posterior attenuation (yellow arrow) are present. C) Image of calcified neoatherosclerosis. Bright protruding tissue with an irregular surface (yellow arrow) is present.
STATISTICAL ANALYSIS
The data are expressed as median (interquartile range). Comparisons between the 2 groups were performed with the Mann-Whitney U test or Fisher’s exact probability test, and differences in means were tested at the critical level of ≤5%. Multivariate analysis was performed with logistic regression. All data were analysed using SPSS software, version 27.0.1.0 (IBM).
Results
This retrospective cross-sectional study conducted at a median of 290 days after DES implantation revealed that NA occurred significantly more often with stents placed in ACS lesions (39% vs 6%; p<0.01) (Table 1). NA was observed in 12 patients, including 3 (4%) in the non-ACS group and 9 (39%) in the ACS group (Central illustration).
In this study, we surveyed 102 patients (23 with ACS and 79 without ACS). All patients had been receiving dual antiplatelet therapy with 2 oral antiplatelet agents for at least 6 months after PCI. The patients’ profiles and main laboratory data are summarised in Table 2. There was no significant difference in the history of PCI, but significantly more patients in the ACS than non-ACS group had a history of ACS (39% vs 11%, respectively; p<0.01).
Table 3 shows the medication and laboratory data at follow-up. Overall, control of the low-density lipoprotein (LDL) cholesterol level was good in both groups. The ACS group received significantly more proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor therapy (p=0.01) and tended to have higher high-sensitivity C-reactive protein levels (p=0.05).
Table 1 shows the target lesion characteristics. The median duration from PCI to follow-up was 287 days in the ACS group and 301 days in the non-ACS group. The ACS group had a higher percentage of patients in whom the right coronary artery was the culprit vessel, but there were no significant differences in lesion characteristics at the time of PCI or during the post-stenting imaging evaluation. Table 4 shows the measurable post-intervention quantitative intravascular imaging data. The distal reference lumen area was significantly lower in the non-ACS group, and the minimum stent area (MSA) tended to be higher in the ACS group. No significant differences were observed in stent expansion or stent landing zone.
We performed univariate and multivariate analyses of factors predicting the occurrence of NA, dividing them into patient background factors and lesion characteristics (Table 5). Both analyses showed a high odds ratio (OR) for stent implantation in patients with ACS. No trends were observed in terms of stent material or drugs.
Regarding the NA characteristics observed on OCT, 10 lesions contained lipid and 2 had calcification. We compared the properties of lipid NA between the ACS and non-ACS groups, but no significant differences were observed (Table 6).
Five of the 12 patients with NA observed during follow-up OCT underwent PCI, but they remained event-free for 3 years.
Table 1. Lesion characteristics.
| Total (n=102) | Non-ACS (n=79) | ACS (n=23) | p-value | |
|---|---|---|---|---|
| Baseline | ||||
| Lesion location | ||||
| LAD | 65 (64) | 52 (66) | 13 (57) | 0.46 |
| LCx | 14 (14) | 12 (15) | 2 (9) | 0.73 |
| RCA | 20 (20) | 12 (15) | 8 (35) | 0.07 |
| Including LMCA | 5 (5) | 5 (6) | 0 | 0.59 |
| Lesion type | ||||
| Type A | 0 (0) | 0 (0) | 0 (0) | |
| Type B1 | 4 (4) | 3 (4) | 1 (4) | 1 |
| Type B2 | 32 (31) | 24 (30) | 8 (35) | 0.8 |
| Type C | 66 (65) | 52 (66) | 14 (61) | 0.81 |
| Chronic total occlusion | 7 (7) | 6 (8) | 1 (4) | 1 |
| Debulking | ||||
| Rotablator | 26 (26) | 20 (25) | 6 (26) | 1 |
| OAS | 4 (4) | 4 (5) | 0 (0) | 0.57 |
| DCA | 2 (2) | 2 (3) | 0 (0) | 1 |
| Type of stent | ||||
| Stent material and drug agent | ||||
| CoCr everolimus-eluting stent | 24 (24) | 20 (25) | 4 (17) | 0.58 |
| Sirolimus-eluting stent | 23 (23) | 19 (24) | 4 (17) | 0.58 |
| PtCr everolimus-eluting stent | 43 (42) | 34 (43) | 9 (42) | 0.81 |
| Zotarolimus-eluting stent | 8 (8) | 4 (6) | 4 (17) | 0.07 |
| Other | 4 (4) | 2 (3) | 2 (9) | 0.22 |
| Drug delivery technology | ||||
| DP-DES | 32 (31) | 24 (30) | 8 (35) | 0.8 |
| BP-DES | 68 (67) | 55 (70) | 13 (57) | 0.31 |
| Other | 2 (2) | 0 (0) | 2 (9) | <0.05 |
| Stent size | ||||
| Stent diameter, mm | 3.00 (2.75-3.50) | 3.00 (2.75-3.50) | 3.00 (3.00-3.50) | 0.06 |
| Stent length, mm | 24 (20-33) | 28 (20-34) | 23 (18-24) | 0.06 |
| Number of stents | 1 (1-1) | 1 (1-1) | 1 (1-1) | 0.82 |
| Total stent length, mm | 28 (23-38) | 32 (23-32) | 24 (20-35) | 0.09 |
| PCI imaging | ||||
| IVUS | 44 (44) | 31 (39) | 13 (57) | 0.49 |
| OCT/OFDI | 57 (56) | 48 (60) | 10 (43) | 0.49 |
| Follow-up | ||||
| Duration, days | 294 (257-337) | 301 (260-339) | 287 (254-321) | 0.41 |
| Neoatherosclerosis | 12 (12) | 3 (4) | 9 (39) | <0.01 |
| Values are presented as n (%) or median with interquartile range (25%-75%). BP-DES: bioabsorbable-polymer drug-eluting stent; CoCr: cobalt-chromium; DCA: directional coronary atherectomy; DP-DES: durable-polymer drug-eluting stent; IVUS: intravascular ultrasound; LAD: left anterior descending artery; LCx: left circumflex artery; LMCA: left main coronary artery; OAS: orbital atherectomy system; OCT: optical coherence tomography; OFDI: optical frequency domain imaging; PCI: percutaneous coronary intervention; PtCr: platinum-chromium; RCA: right coronary artery | ||||
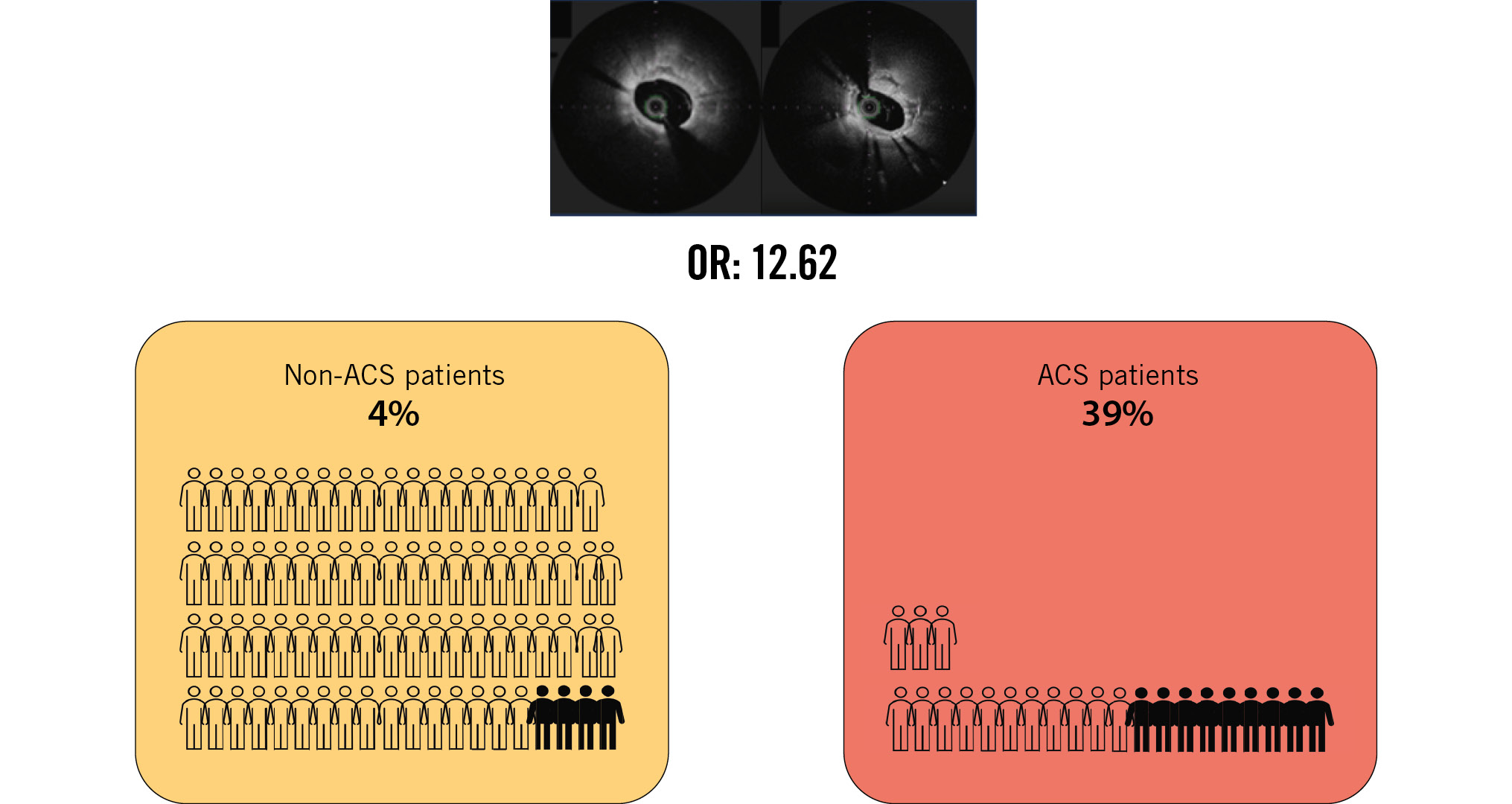
Central illustration. ACS is an independent predictor of neoatherosclerosis one year after 2nd-generation DES implantation. ACS: acute coronary syndrome; DES: drug-eluting stent; OR: odds ratio
Table 2. Baseline clinical characteristics.
| Total (n=102) | Non-ACS (n=79) | ACS (n=23) | p-value | |
|---|---|---|---|---|
| Age, years | 71 (65-78) | 71 (65-77) | 74 (69-80) | 0.19 |
| Male | 79 (77) | 64 (81) | 15 (65) | 0.16 |
| Hypertension | 75 (74) | 60 (76) | 15 (65) | 0.42 |
| Hypercholesterolaemia | 74 (73) | 58 (73) | 16 (70) | 0.79 |
| Diabetes mellitus | 41 (40) | 34 (43) | 7 (30) | 0.34 |
| Haemodialysis | 6 (6) | 3 (4) | 3 (13) | 0.13 |
| Previous coronary angioplasty | 31 (30) | 25 (30) | 6 (30) | 0.8 |
| Previous coronary artery bypass | 2 (2) | 1 (1) | 1 (4) | 0.4 |
| Previous ACS | 18 (17) | 9 (11) | 9 (39) | <0.01 |
| CPA | 3 (3) | 2 (3) | 1 (4) | 0.54 |
| Medication | ||||
| Statin | 56 (55) | 46 (58) | 10 (43) | 0.24 |
| ACEi or ARB | 49 (48) | 40 (51) | 9 (39) | 0.35 |
| PCSK9 inhibitor | 2 (2) | 0 (0) | 2 (9) | 0.05 |
| Angina status | ||||
| Stable | 79 (77) | |||
| Acute coronary syndrome | 23 (21) | 23 (100) | ||
| STEMI | 8 (8) | 8 (35) | ||
| NSTEMI or UAP | 15 (15) | 15 (65) | ||
| Laboratory data | ||||
| WBC, /μL | 6,671 (5,200-7,400) | 6,300 (5,200-7,500) | 5,900 (5,000-7,200) | 0.72 |
| Hb, g/dL | 13.4 (12.5-14.7) | 13.7 (12.6-15.0) | 12.7 (11.6-14.3) | 0.22 |
| hs-CRP, mg/dL | 0.11 (0.05-0.29) | 0.11 (0.05-0.29) | 0.07 (0.04-0.57) | 0.73 |
| BUN, mg/dL | 17.5 (14-23) | 18 (14-23) | 17 (14-29) | 0.9 |
| Creatinine, mg/dL | 0.9 (0.8-1.1) | 0.9 (0.76-1.09) | 0.87 (0.75-1.23) | 0.95 |
| eGFR, ml/min/1.73m2 | 61 (48-74) | 61 (49-74) | 58 (32-69) | 0.42 |
| UA, mg/dL | 5.8 (4.8-6.7) | 5.9 (4.9-6.8) | 5.0 (4.2-6.1) | 0.37 |
| Triglyceride, mg/dL | 110 (86-174) | 110 (87-184) | 125 (62-164) | 0.67 |
| Total cholesterol, mg/dL | 164 (136-188) | 162 (136-180) | 178 (146-208) | 0.24 |
| HDL cholesterol, mg/dL | 49 (39-59) | 47 (38-57) | 51 (42-60) | 0.7 |
| LDL cholesterol, mg/dL | 88 (73-117) | 88 (70-114) | 100 (75-125) | 0.24 |
| Non-HDL cholesterol, mg/dL | 114 (91-142) | 109 (90-132) | 125 (97-152) | 0.13 |
| FBS, mg/dL | 103 (94-129) | 101 (94-125) | 110 (93-136) | 0.18 |
| HbA1c, % | 6.0 (5.7-6.6) | 6.0 (5.7-6.4) | 6.1 (5.6-7.0) | 0.2 |
| hs-cTnT, ng/mL | 15 (8-30) | 10 (8-16) | 19 (8-39) | 0.2 |
| BNP, pg/mL | 46 (21-120) | 44 (17-92) | 51 (25-51) | 0.17 |
| Lp(a), mg/dL | 11 (5-15) | 11 (5-14) | 15 (7-39) | 0.11 |
| Values are presented as n (%) or median with interquartile range (25%-75%). ACEi: angiotensin-converting enzyme inhibitor; ACS: acute coronary syndrome; ARB: angiotensin II receptor blocker; BNP: brain natriuretic peptide; BUN: blood urea nitrogen; CPA: cardiopulmonary arrest; eGFR: estimated glomerular filtration rate; FBS: fasting blood sugar; Hb: haemoglobin; HbA1c: haemoglobin A1c; HDL: high-density lipoprotein; hs-CRP: high-sensitivity C-reactive protein; hs-cTnT: high-sensitivity cardiac troponin T; LDL: low-density lipoprotein; Lp: lipoprotein; PCSK9: proprotein convertase subtilisin/kexin type 9; NSTEMI: non-ST-segment elevation myocardial infarction; STEMI: ST-segment elevation myocardial infarction; UA: uric acid; UAP: unstable angina pectoris; WBC: white blood cells | ||||
Table 3. Medication and laboratory data at follow-up.
| Total (n=102) | Non-ACS (n=79) | ACS (n=23) | p-value | |
|---|---|---|---|---|
| Medication | ||||
| Statin | 94 (92) | 74 (94) | 20 (87) | 0.38 |
| ACEi/ARB | 60 (59) | 49 (62) | 11 (48) | 0.24 |
| PCSK9 inhibitor | 8 (8) | 3 (4) | 5 (22) | 0.01 |
| Laboratory data | ||||
| WBC, /μL | 6,000 (5,075-7,000) | 6,000 (5,100-7,075) | 5,650 (4,275-6,300) | 0.43 |
| Hb, g/dL | 13.5 (12.2-14.5) | 13.5 (12.2-14.6) | 12.9 (12.2-14.5) | 0.58 |
| hs-CRP, mg/dL | 0.07 (0.03-0.16) | 0.06 (0.03-0.110) | 0.11 (0.04-0.55) | 0.05 |
| BUN, mg/dL | 18 (15-22) | 18 (15-22) | 22 (18- 26) | 0.72 |
| Creatinine, mg/dL | 0.9 (0.8-1.1) | 0.9 (0.7-1.1) | 0.9 (0.7-4.5) | 0.9 |
| eGFR, ml/min/1.73 m2 | 62 (48-73) | 60 (50-73) | 55 (15-76) | 0.41 |
| UA, mg/dL | 5.4 (4.4-6.4) | 5.2 (4.5-6.6) | 4.8 (3.8-6.0) | 0.1 |
| Triglyceride, mg/dL | 109 (79-168) | 103 (80-171) | 118 (88-178) | 0.84 |
| Total cholesterol, mg/dL | 144 (126-163) | 138 (123-156) | 145 (135-167) | 0.31 |
| HDL cholesterol, mg/dL | 52 (40-60) | 51 (39-59) | 53 (44-62) | 0.45 |
| LDL cholesterol, mg/dL | 67 (55-82) | 66 (52-80) | 67 (61-86) | 0.61 |
| Non-HDL cholesterol, mg/dL | 95 (79-106) | 93 (73-104) | 98 (85-104) | 0.52 |
| FBS, mg/dL | 103 (92-122) | 104 (90-121) | 126 (92-134) | 0.74 |
| HbA1c, % | 6.1 (5.8-6.7) | 6.1 (5.8-6.8) | 6.3 (5.7-7.4) | 0.79 |
| BNP, pg/mL | 32 (15-65) | 39 (13-76) | 31 (16-134) | 0.46 |
| Lp(a), mg/dL | 11 (5-20) | 10 (4-20) | 18 (6-44) | 0.14 |
| Values are presented as n (%) or median with interquartile range (25%-75%). ACEi: angiotensin-converting enzyme inhibitor; ACS: acute coronary syndrome; ARB: angiotensin II receptor blocker; BNP: brain natriuretic peptide; BUN: blood urea nitrogen; eGFR: estimated glomerular filtration rate; FBS: fasting blood sugar; Hb: haemoglobin; HbA1c: haemoglobin A1c; HDL: high-density lipoprotein; hs-CRP: high-sensitivity C-reactive protein; LDL: low-density lipoprotein; Lp: lipoprotein; NSTEMI: non-ST-segment elevation myocardial infarction; PCSK9: proprotein convertase subtilisin/kexin type 9; STEMI: ST-segment elevation myocardial infarction; UA: uric acid; WBC: white blood cells | ||||
Table 4. Post-intervention quantitative intravascular imaging data.
| Total (n=97) |
Non-ACS (n=77) |
ACS (n=20) |
p-value | |
|---|---|---|---|---|
| Distal reference lumen area, mm2 | 5.71 (4.37-7.78) | 5.41 (4.08-7.18) | 6.57 (4.93-8.85) | 0.04 |
| Proximal reference lumen area, mm2 | 8.23 (6.35-9.70) | 8.26 (6.35-9.78) | 7.67 (5.98-9.23) | 0.74 |
| MSA, mm2 | 5.40 (3.84-6.22) | 5.12 (3.71-6.07) | 5.80 (4.65-7.94) | 0.06 |
| Conventional stent expansion, % | 75.5 (64.7-85.4) | 75.1 (64.0-84.5) | 82.3 (70.0-90.3) | 0.58 |
| MSA by distal reference lumen area, % | 93.9 (79.6-102.9) | 93.9 (80.8-104.2) | 94.8 (78.5-100.2) | 0.1 |
| Malapposition | 21 (22) | 17 (22) | 4 (20) | 0.19 |
| Protrusion | 21 (22) | 14 (18) | 7 (35) | 0.79 |
| Landing zone disease | ||||
| Edge dissection | 20 (21) | 18 (23) | 2 (10) | 0.73 |
| On the calcified lesion | 20 (21) | 12 (16) | 8 (40) | 0.6 |
| Values are presented as n (%) or median with interquartile range (25%-75%). Conventional stent expansion is calculated as follows: MSA/average reference lumen area×100 (with the average reference lumen area calculated as follows: 1/2 [proximal reference lumen area+distal reference lumen area]). MSA by distal reference lumen area is calculated as MSA/distal reference lumen area×100. ACS: acute coronary syndrome; MSA: minimum stent area | ||||
Table 5. Univariate and multivariate analyses to predict neoatherosclerosis based on patient and lesion characteristics at follow-up.
| Univariate | Multivariate model 1 | Multivariate model 2 | Multivariate model 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | pvalue | OR | 95% CI | p-value | |
| Stent implantation for ACS | 16.29 | 3.92-67.75 | <0.01 | 17.11 | 4.03-72.64 | <0.01 | 12.62 | 2.89-54.99 | <0.01 | 15.12 | 3.53-64.78 | <0.01 |
| Age | 1 | 0.94-1.06 | 0.87 | 0.98 | 0.92-1.05 | 0.56 | ||||||
| LDL cholesterol | 1.02 | 0.99-1.04 | 0.24 | |||||||||
| Statin | 0.36 | 0.06-2.01 | 0.24 | |||||||||
| PCSK9 inhibitor | 8.09 | 0.47-138.72 | 0.15 | |||||||||
| hs-CRP | 3.51 | 1.18-10.44 | 0.02 | 2.24 | 0.70-7.52 | 0.18 | ||||||
| eGFR | 0.97 | 0.95-1.00 | 0.03 | 0.98 | 0.95-1.01 | 0.1 | ||||||
| Stent diameter | 0.7 | 0.19-2.55 | 0.58 | |||||||||
| Stent length | 0.98 | 0.91-1.05 | 0.55 | |||||||||
| Total stent length | 0.98 | 0.94-1.03 | 0.46 | |||||||||
| RCA lesion | 0.8 | 0.16-3.98 | 0.79 | |||||||||
| Duration of follow-up | 1 | 0.99-1.01 | 0.88 | |||||||||
| DP-DES | 1.11 | 0.31-3.98 | 0.88 | |||||||||
| BP-DES | 1 | 0.28-3.59 | 1 | |||||||||
| Zotarolimus-eluting stent | 1.08 | 0.12-9.61 | 0.95 | |||||||||
| Distal reference lumen area | 1 | 0.77-1.28 | 0.98 | |||||||||
| MSA | 0.93 | 0.68-1.29 | 0.68 | |||||||||
| ACS: acute coronary syndrome; BP-DES: bioabsorbable-polymer drug-eluting stent; CI: confidence interval; DP-DES: durable-polymer drug-eluting stent; eGFR: estimated glomerular filtration rate; hs-CRP: high-sensitivity C-reactive protein; LDL: low-density lipoprotein; MSA: minimum stent area; OR: odds ratio; PCSK9: proprotein convertase subtilisin/kexin type 9; RCA: right coronary artery | ||||||||||||
Table 6. OCT/OFDI analyses of lipid neoatherosclerosis.
| Total (n=10) |
Non-ACS (n=3) | ACS (n=7) |
p-value | |
|---|---|---|---|---|
| Max lipidarc, ° | 138 | 108 | 152 | 0.52 |
| Lipid core length, mm | 5.6 | 5.8 | 5.3 | 0.83 |
| Lipid volume index | 448 | 422 | 475 | 1 |
| Values are presented as median. ACS: acute coronary syndrome; OCT: optical coherence tomography; OFDI: optical frequency domain imaging | ||||
Discussion
This study showed that NA occurred more frequently in ACS lesions than in non-ACS lesions at approximately 1 year of follow-up.
This retrospective cross-sectional study used the occurrence of NA as an outcome. By contrast, other stent follow-up OCT observational studies used clinically driven target lesion revascularisation or clinically driven target vessel revascularisation as an outcome. The results of the present study are considered useful for understanding the process of intimal repair after DES implantation.
In approximately 64% of cases, ACS is caused by thrombosis due to plaque rupture and subsequent platelet aggregation, and this is followed by plaque erosion in 25% of cases. ACS caused by calcified nodules has also recently received attention. In any case, the characteristics of the lesions that cause ACS are different from the characteristics of stable coronary artery lesions6.
Regarding lipid management, effective control of LDL cholesterol using potent statin therapy is recommended for secondary prevention after PCI378. In the present study, PCI was performed with an LDL cholesterol level of 88 mg/dL and well-controlled lipids. At follow-up, the LDL cholesterol level was controlled at <70 mg/dL in both groups, and PCSK9 inhibitors were also aggressively administered in high-risk patients. Although there is no clear evidence that the LDL cholesterol level is involved in the development of NA, a high LDL cholesterol level has been reported to be an independent predictor of NA incidence and plaque vulnerability in patients with late ISR9, and LDL cholesterol management is important after PCI. In LDL cholesterol management, a 50% reduction is recommended for secondary prevention of atherosclerotic cardiovascular disease in the USA7, and a level of <50 mg/dL is recommended in Europe8. In the present study, the median LDL cholesterol level was 67 mg/dL (interquartile range [IQR] 55-82 mg/dL), and the reduction rate was 22% (IQR 0-43%), which may have left room for further therapeutic intervention; however, no correlation was found with the occurrence of NA.
OCT is one of the best intravascular imaging techniques for the diagnosis of NA because of its very high resolution and excellent assessment of lesion characteristics5. Gonzalo et al10 initially classified OCT images with different ISR patterns as layered, homogeneous, and heterogeneous, and Yamamoto et al11 further classified them into 6 categories. In one study, OCT images of ISR showed more layered and heterogeneous patterns in DES and more homogeneous patterns in BMS12, suggesting that the neointima of DES and BMS are pathologically different. In a pathological study, Nakazawa et al13 suggested that DES implantation is strongly associated with the development of NA, whereas patients with BMS implantation rarely develop NA <3 years after implantation but often develop NA >6 years later. NA progression is accelerated in patients who undergo DES implantation <2 years after PCI813, and this may be a cause of thrombosis. An observational OCT study of ISR lesions by Nakamura et al14 also showed that ISR of DES, including first-generation DES, was an independent predictor of NA. With regard to second-generation DES, a study comparing the lesion characteristics of early (<1 year) and late (>1 year) restenosis showed a higher frequency of uniform neointima in the early group and a significantly higher frequency of lipid-laden thin-cap fibroatheroma, neovascularisation, and macrophage infiltration in the late group15. These changes were thought to be due to the delayed arterial healing associated with DES implantation, even with second-generation DES implantation. Most of these previous studies involved lesions that had undergone stenting followed by either clinically driven target lesion revascularisation or clinically driven target vessel revascularisation. However, ours was a cross-sectional study involving the evaluation of lesions at 1 year after implantation of new-generation DES, and our findings may help to elucidate the mechanisms underlying the intimal repair process after DES implantation in ACS lesions.
A similar observational OCT study was performed 1 year after durable-polymer DES and bioabsorbable-polymer DES implantation. The study showed no statistically significant differences between the 2 types of DES, but treatment with renin-angiotensin system (RAS) inhibitors reduced the risk of NA16. Additionally, the study did not show that unstable angina or myocardial infarction were predictive factors for the incidence of NA, but this may have been due to differences in the patients’ backgrounds and the study design. Regarding the effect of RAS inhibitors on the incidence of NA, the present study showed the opposite trend (OR 2.39; p=0.18). This trend might be explained by our very high-risk patient population or a possible residual risk of NA occurrence that cannot be prevented with RAS inhibitors.
The finding that NA can occur 1 year after DES implantation, even with thorough secondary prevention, indicates that there are still unknown residual risk factors in patients with ACS. Thus, further stent drug delivery technologies need to be developed for ACS lesions.
Limitations
This study had several limitations. First, it was a retrospective, single-centre, observational study. Second, the number of patients with ACS was small. Third, because IVUS was included as an intravascular imaging technique at the time of PCI, measurement errors between OCT and IVUS may have occurred in the assessment of stent malapposition and protrusion immediately after treatment. Fourth, not all post-PCI patients were followed up, resulting in a possible selection bias.
Conclusion
The results of this observational study of OCT follow-up examination after second-generation DES implantation showed that ACS lesions were associated with the development of early NA.
Impact on daily practice
Stent thrombosis due to early neoatherosclerosis is a concern even with the latest generation of drug-eluting stents. The results of this study suggest that stent implantation for acute coronary syndrome lesions is a risk factor for early neoatherosclerosis occurrence. These results may inform future strategies for percutaneous coronary intervention and the development of stents tailored to acute coronary syndrome.
Acknowledgements
We thank Edanz for editing a draft of this manuscript.
Conflict of interest statement
The authors have no conflicts of interest to declare.
