Introduction
Infection of a coronary stent is a rare complication associated with percutaneous coronary intervention (PCI), and only a few cases have been reported in the literature1. This complication can manifest in various ways, such as the formation of a mycotic coronary artery aneurysm, a pseudoaneurysm, a myocardial abscess, or pericarditis with exudative pericardial effusion234567. Notably, a coronary artery pseudoaneurysm represents the most common presentation8. However, managing this condition presents significant challenges and carries the potential for lethality, even in specialised centres9. Given the paucity of literature, we seek to assess the clinical features, management strategies, and outcomes of patients presenting with stent infection following percutaneous coronary angioplasty at a major referral centre in India between 2018 and 2022.
Objectives
The objective of the study was to describe the clinical features, patient and procedural factors, management strategies and outcomes of patients presenting with stent infection following PCI.
Methods
Consecutive patients with coronary stent infection between 2018 and 2022 at a single tertiary care teaching hospital were retrospectively included in this study. The research was conducted after obtaining clearance from the Institutional Ethics Committee. Patients were considered to have possible coronary stent infection if they presented with a history of prolonged fever (>7 days) following PCI. If the initial evaluation did not reveal any obvious cause for the fever, the patient underwent further workup for stent infection, as shown in Figure 1. Additionally, patients presenting without an obvious history of fever but with a repeat angiogram done for the evaluation of recurrence of chest pain that showed an aneurysm or pseudoaneurysm in or around the stented segment and those presenting with pericardial effusion within 30 days of PCI were also considered to have probable coronary stent infection and were subjected to further investigation. All patients who met the criteria for “probable stent infection” were included in the final analysis. Among these, patients who had undergone surgery for the removal of the stent, showing histopathological or microbiological evidence of infection, were classified as having “definite stent infection”.
Data on procedure- and centre-related factors at the time of the initial PCI were extracted from electronic and paper charts. Procedure-related factors included the nature of the procedure (emergency vs elective), history of preceding recent febrile illness, vascular access (radial vs femoral), history of multiple punctures of the same site within a week, and the use of multiple devices, including intravascular imaging and flow analysis devices, rotablation, and circulatory support devices. Centre-related factors included the average number of interventional procedures (PCIs) performed in a month; the age of the cath lab; reuse of hardware such as sheaths, wires, and balloons involved in the procedure after sterilisation; the average duration of removal of the vascular access sheath after the completion of the procedure; and the practice of regular microbiological surveillance in the cath lab. The experience of the primary operator, both in years and in the volume of cases performed, was also included. Since most patients underwent the index PCIs at different health centres, these data were collected by reviewing discharge summaries and through telephone interviews. Imaging studies, including transthoracic (TTE) and transoesophageal echocardiograms (TOE), computed tomography (CT) cardiac angiograms, and positron emission tomography (PET)-CT scans, were also reviewed, and relevant data were collected. Lab tests, including inflammatory markers and renal and liver function tests, were drawn in all patients upon admission and serially. Blood cultures (3 sets) were obtained upon admission, and all patients were started on empirical antibiotics. Blood cultures were repeated if clinically indicated. In cases of culture-negative results, broad-spectrum antibiotics were initiated after consultation with the Infectious Diseases department. The decision on patient management, whether medical or surgical intervention, was made after discussion in a multidisciplinary team, including a cardiologist, an infectious disease specialist, and a cardiothoracic surgeon.
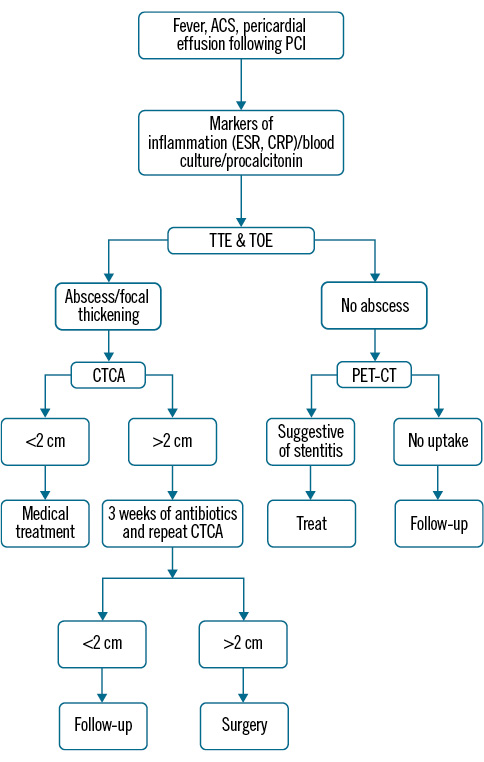
Figure 1. Diagnostic algorithm. ACS: acute coronary syndrome; CRP: C-reactive protein; CT: computed tomography; CTCA: computed tomography coronary angiography; ESR: erythrocyte sedimentation rate; PCI: percutaneous coronary intervention: PET: positron emission tomography; TOE: transoesophageal echocardiogram; TTE: transthoracic echocardiogram
Statistical analysis
Data are presented as numbers and percentages for categorical variables and as mean and standard deviation or median and interquartile range for continuous variables. Predictors of outcomes could not be studied because of the small sample size.
Results
A total of 11 patients with probable stent infections were included. The majority were male (7, 63.6%), with a mean age at presentation of 55.6±7.2 years. Five patients (45.6%) had hypertension, and 2 (18.2%) had diabetes. Two (18.2%) had single-vessel coronary artery disease (CAD), and 9 (81.8%) had multivessel CAD. PCI was performed in the right coronary artery (RCA) in 6 patients (63.6%), while it was performed in the left anterior descending artery (LAD) for 2 patients (18.2%) and in the left circumflex artery (LCx) for 3 patients (27.3%). None of the patients underwent multivessel PCI. Imaging modalities such as optical coherence tomography (OCT)/intravascular ultrasound (IVUS), fractional flow reserve (FFR) physiological assessment, or plaque modification devices like rotablator were not utilised during any of the PCI procedures. Additionally, no patients received mechanical circulatory support during the procedure.
Procedural and patient factors
Regarding procedural and patient factors, 7 patients underwent the procedure via the femoral route, while 4 underwent it via the radial route. Three cases reported repeat femoral puncture within a week. Hardware reuse was reported in 6 cases, 1 case reported no reuse of hardware, and data on reuse could not be obtained in 4 cases. The duration of the procedure was less than 30 minutes in 4 cases and more than 30 minutes in 7 cases. Three patients reported preceding febrile illness before coronary intervention. Sheath removal occurred after 4 hours in 3 cases. Microbiological surveillance every 6 months was lacking in 5 catheterisation labs, and data could not be obtained from 3 centres.
Presentation
Fever was the most common symptom at presentation, present in 8 (72.7%) patients, with the majority experiencing its onset within a week of PCI. Two patients presenting with pericardial tamponade developed fever during hospitalisation. Four patients had pericardial effusion, and 3 developed cardiac tamponade, necessitating emergent pericardiocentesis. Baseline characteristics are summarised in Table 1. The median time to the development of effusion was 3 days. In 1 case, presentation occurred as epigastric swelling 7 years after angioplasty. Evaluation via CT revealed a massive abscess originating from an RCA stent and eroding the diaphragm. This patient had fever following the index procedure and received multiple courses of antibiotics, including ceftriaxone, ciprofloxacin, and amoxicillin-clavulanic acid. Blood cultures drawn at the time returned negative.
Table 1. Baseline characteristics.
| Characteristic | n=11 |
|---|---|
| Age, years | 55.6 (48-65) |
| Sex, male | 7 (63.6) |
| DM | 2 (18.2) |
| Indication for angioplasty | |
| STEMI | 7 (63.6) |
| NSTEMI | 2 (18.2) |
| Stable IHD | 2 (18.2) |
| Time after angioplasty until presentation, days | 6 (3-14)* |
| Fever | 8 (72.7) |
| Pericardial effusion | 4 (36.4) |
| Tamponade | 3 (27.3) |
| Total leukocyte count, cc/mm3 | 11,820 (9,800-13,800) |
| ESR, mm/hr | 108 (82-130) |
| CRP, mg% | 142 (34-229) |
| Creatinine, mg% | 1.2 (0.9-7.2) |
| Infected vessel | |
| RCA | 6 (54.5) |
| LCx | 3 (27.3) |
| LAD | 2 (18.2) |
| Stent type | |
| SE | 6 (54.5) |
| EE | 5 (45.5) |
| Culture | |
| Staphylococcus aureus | 6 (54.5) |
| Pseudomonas aeruginosa | 2 (18.2) |
| Negative | 3 (27.3) |
| Values are median (interquartile range) or n (%). *One outlier at 7 years. CRP: C-reactive protein; DM: diabetes mellitus; EE: everolimus-eluting; ESR: erythrocyte sedimentation rate; IHD: ischaemic heart disease; LAD: left anterior descending artery; LCx: left circumflex artery; NSTEMI: non-ST-segment elevation myocardial infarction; RCA: right coronary artery; SE: sirolimus-eluting; STEMI: ST-segment elevation myocardial infarction | |
Investigations
Laboratory investigations revealed elevated erythrocyte sedimentation rate (ESR; median 108 mm/hr) and C-reactive protein (CRP; median 142 mg/L) in all patients at presentation. The total white blood cell count was mildly elevated in 5 cases, and peripheral smear showed a left shift and toxic granules in 6 cases. One patient presented with acute kidney injury, with a serum creatinine of 7.2 g/dL. Liver function tests were normal in all patients at presentation. The organism identified was Staphylococcus aureus in 6 cases, followed by Pseudomonas aeruginosa in 2 cases, with the remaining 3 cases yielding negative cultures. Individual case details are summarised in Table 2.
Imaging studies involving TTE on admission displayed an abscess cavity in 10 cases (6 in the right atrioventricular [AV] groove, 3 in the left AV groove, and 1 in the left ventricular free wall) (Figure 2A–Figure 2D, Moving image 1, Moving image 2). In 1 case, the abscess cavity became visible on serial TTE 2 weeks after presentation. A TOE was performed in all cases upon admission, with repeat TOE done at 2-week intervals for medically managed cases. TTE and TOE features of the stent abscess included an echolucent space with a thick, well-defined capsule in the majority of cases, ranging in size from 1.8 cm to 9 cm. One case, showing a highly reflective stent inside the radiolucent cavity, is illustrated in Figure 2C (Moving image 3). In the 10th case, the capsule was ill defined, and a huge multiloculated irregular mass was observed near the right atrial free wall and AV groove. In patients presenting with pericardial effusion and tamponade, the aneurysms exceeded 5 cm in size. Even in the case where the abscess cavity was not initially obvious on TTE, focal thickening suggesting early infection was apparent in the left ventricular free wall (Figure 3A, Moving image 4, Moving image 5). In 1 patient, the abscess in the RCA (AV groove) compressed the tricuspid valve annulus causing significant obstruction to tricuspid inflow (Figure 3B–Figure 3D, Moving image 6, Moving image 7). In another patient, the abscess size rapidly increased after 2 weeks of antibiotics, leading to surgical intervention. Unfortunately, this patient developed septic shock after surgery and passed away on postoperative day 3.
CT coronary angiography (CTCA) was performed in 7 cases, while it was deferred in 4 cases due to renal impairment. CTCA provided a clear view of the abscess location and morphology, correlating well with echocardiogram findings (Figure 4A–Figure 4B). Among the 7 patients who underwent CTCA, 6 underwent a repeat CT during treatment to assess the size of the aneurysm under medical management. In 4 patients, the abscess size remained unchanged, while in 2 patients the abscess size significantly increased. One patient underwent PET-CT, which showed focal increased fluorodeoxyglucose (FDG) uptake along the stent in the LCx territory, suggesting infection (Figure 4C). Imaging details of the cases are summarised in Table 3.
Invasive coronary angiography was performed in 2 patients who presented with features of acute coronary syndrome (ACS; chest pain and elevated troponin). Both patients had undergone LCx PCI recently (Figure 5). One patient displayed a clear aneurysm of the stented segment and was advised surgery but refused it. A repeat angiogram after completing a 4-week antibiotic treatment with vancomycin and piperacillin-tazobactam showed complete resolution of the aneurysm. The other patient had proximal occlusion of the vessel, and the stented segment was not visible during the procedure.
Table 2. Description of cases.
| Case | Age & sex | Stented artery | Stent type | Indication for initial PCI | Presenting symptom/ syndrome | Timing of fever | Organism cultured | Systems involved | Treatment | Course/ outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 48 M | RCA | Sirolimus | ACS STEMI | Fever | Day 4 | Negative | CV | Surgery | Survived |
| 2 | 51 M | RCA | Sirolimus | ACS STEMI | Epigastric swelling | No fever | Staphylococcus aureus | CV, abdominal | Surgery | Survived |
| 3 | 55 M | LCx LAD | Everolimus | ACS NSTEMI | Fever, pericarditis | Day 5 | Staphylococcus aureus | CV | Surgery | Expired |
| 4 | 56 F | LAD LCx RCA | Everolimus | CSA | Fever, pericarditis,tamponade | Day 3 | Staphylococcus aureus | CV | Medical | Survived |
| 5 | 53 M | RCA | Everolimus | ACS STEMI | Fever, tamponade | Day 11 | Staphylococcus aureus | CV | Medical | Survived |
| 6 | 55 F | LAD | Sirolimus | CSA | Tamponade | Day 3 | Pseudomonas aeruginosa | CV, renal | Surgery | Expired |
| 7 | 56 F | RCA | Sirolimus | ACS STEMI | Fever | Day 7 | Staphylococcus aureus | CV | Medical | Survived |
| 8 | 65 F | RCA LCx | Everolimus | ACS STEMI | Fever,acute stent thrombosis | Day 6 | Staphylococcus aureus | CV | Surgery | Survived |
| 9 | 60 M | RCA LAD | Sirolimus | ACS NSTEMI | Fever, pericarditis | Day 5 | Negative | CV | Surgery | Survived |
| 10 | 55 M | RCA | Everolimus | ACS IWMI | Tamponade | Day 9 | Pseudomonas aeruginosa | CV | Surgery | Survived |
| 11 | 58 M | LCx | Sirolimus | STEMI | Fever | Day 13 | Negative | CV | Medical | Survived |
| ACS: acute coronary syndrome; CSA: chronic stable angina; CV: cardiovascular; F: female; IWMI: inferior wall myocardial infarction; LAD: left anterior descending artery; LCx: left circumflex artery; M: male; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; RCA: right coronary artery; STEMI: ST-segment elevation myocardial infarction | ||||||||||
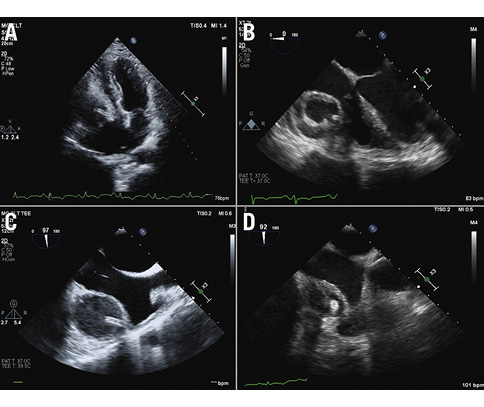
Figure 2. Echocardiographic characteristics. A) A transthoracic echo showing a stent abscess with the linear shadow of the stent inside; B) transoesophageal echo (TOE) showing a stent abscess of the right coronary artery with the thick wall of the cavity; C) TOE showing the linear shadow of the stent; D) TOE showing the multiloculated appearance of the stent abscess
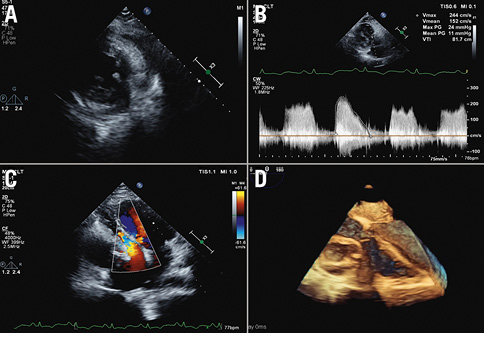
Figure 3. Echocardiographic characteristics. A) Localised thickening visible in the lateral wall of the left ventricle in the early phase of the stent; B) continuous wave Doppler through the TV showing a significant gradient across the TV; C) abscess compressing the right ventricle, right atrium and TV; D) 3D TOE showing the stent abscess distorting the TV. 3D: three-dimensional; TOE: transoesophageal echocardiogram; TV: tricuspid valve
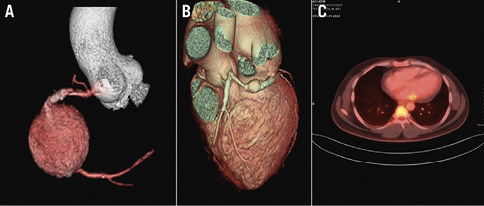
Figure 4. CT and PET-CT characteristics. A) CAG showing a large pseudoaneurysm at the stented segment of the RCA; B) a view of the pseudoaneurysm from the stented segment of the LCx; C) PET-CT showing focal increased uptake along the stent in LCx territory. No other focal abnormal hypermetabolism was noted elsewhere in the myocardium. CAG: coronary angiogram; CT: computed tomography; LCx: left circumflex artery; PET: positron emission tomography; RCA: right coronary artery
Table 3. Imaging details.
| Case | TTE | TOE | CT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Size of abscess, cm | Maximum thickness of capsule, mm | Septations | Structures compressed or distorted | Maximum size,cm | Stent visibility inside | Valvular regurgitation | Maximum diameter, cm | Serial increase in repeat CT | Myocardial abscess or extension | |
| 1 | 4.8 | 3 | Nil | TV annulus | 4.9 | Yes | Nil | 5.0 | No | No |
| 2 | 9 | 4 | Nil | TV | 12 | Yes | Nil | 11 | No | No |
| 3 | 3 | 2 | Nil | Nil | 3.4 | Yes | Nil | 4.2 | Yes | No |
| 4 | 4 | 3 | Nil | Nil | 4.2 | No | Nil | ND | ND | ND |
| 5 | 1.5 | – | Nil | Nil | 1.8 | No | Nil | ND | ND | ND |
| 6 | 5.3 | 4 | Yes | TV | 5.5 | Yes | Nil | 6.7 | Yes | No |
| 7 | 1.6 | – | Nil | Nil | 1.5 | Nil | Nil | ND | ND | ND |
| 8 | 6 | 3.4 | Nil | Nil | 5 | Yes | Nil | 5.5 | No | No |
| 9 | 7 | 4 | Nil | Nil | 7.4 | Yes | Nil | 7.5 | ND | No |
| 10 | 10.4 | 3 | Yes | TV | 12 | Yes | Nil | 8 | No | Yes |
| 11 | 1.6 | – | Nil | Nil | 1.7 | Yes | Nil | 1.9 | ND | No |
| CT: computed tomography; ND: no data; TOE: transoesophageal echocardiogram; TTE: transthoracic echocardiogram; TV: tricuspid valve | ||||||||||
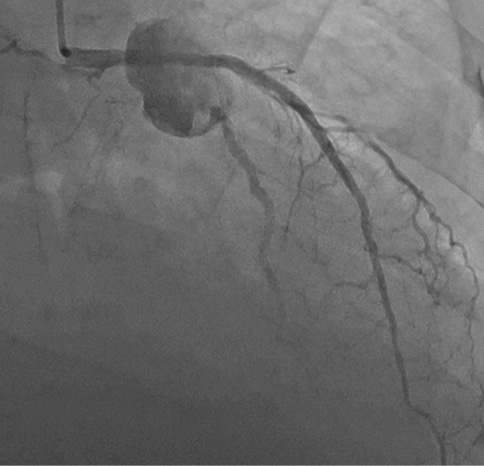
Figure 5. Angiogram showing an aneurysm of a proximal left circumflex artery.
Management
All patients received empirical broad-spectrum antibiotics once the diagnosis was made, with adjustments based on culture and sensitivity reports. Following multidisciplinary team discussions, surgery was contemplated for all 7 patients with abscesses exceeding 2 cm. One patient declined surgery and underwent medical management; she experienced resolution of the infection and aneurysm following 6 weeks of antibiotic therapy. The other 6 patients underwent surgery within 3 weeks. Five of these patients survived to hospital discharge, while 1 experienced postoperative mortality on postoperative day 3. Another patient, initially planned for medical management due to high operative risk and frailty, exhibited a rapid increase in abscess size on 1-week follow-up echo and underwent emergency surgery, ultimately passing away on postoperative day 2 due to refractory septic shock. Three patients with relatively smaller aneurysms, measuring less than 2 cm, were managed medically. All 3 survived to hospital discharge and completed 4 weeks of antibiotics without fever recurrence. Subsequent TOE did not reveal a persistent abscess, and none required repeat CT.
Surgery for stent abscesses posed significant challenges and was performed by highly experienced surgeons (Moving image 8– shows intraoperative stent extraction). Abscesses were visible upon pericardium opening in 6 cases (Figure 6), with 1 case showing only inflamed tissue. After abscess excision, saphenous vein grafting was performed distally in all cases. In 1 case with pus tracking towards the epigastrium and eroding the diaphragm, the abscess was aspirated even before the sternotomy due to impending rupture. The treatment protocol is summarised in the flowchart (Figure 7).
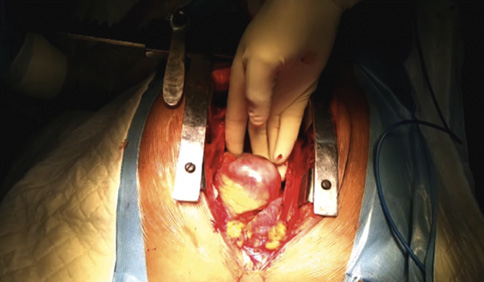
Figure 6. Intraoperative picture showing a large abscess of a right coronary artery.
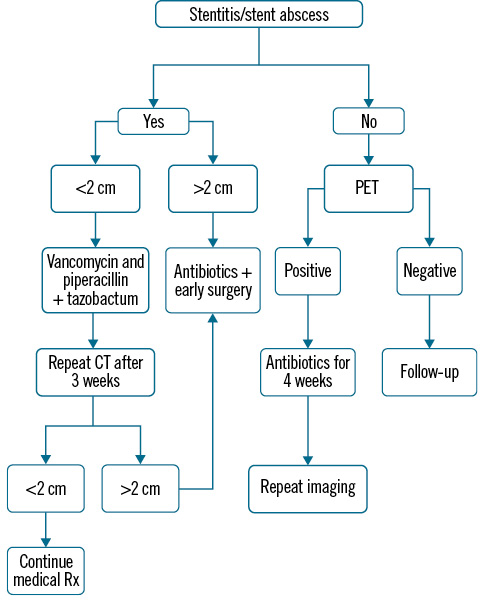
Figure 7. Treatment algorithm. CT: computed tomography; PET: positron emission tomography; Rx: prescription
Discussion
Since the first reported case of coronary stent infection in 199310, fewer than 80 cases have been documented worldwide11. Endothelial damage in coronary arteries can result from both balloon dilatation and metal stent implantation, enabling bacteria to penetrate the vessel wall through compromised endothelial layers, which can occur during stent implantation. The stent itself can serve as a nidus for pathogen adherence, exacerbating the risk of infection12. Stent infections have been observed with both bare metal stents (BMS) and drug-eluting stents (DES), but in our study, all patients had undergone angioplasty with DES implantation.
The incidence of stent infection has risen since the introduction of DES in 2003. Of the 24 reported cases of infected coronary artery stents since then, 16 involved DES implantation, while only 8 involved BMS implantation11. Neointima formation after stenting may act as a protective barrier against infection13. However, DES can interfere with neointimal growth due to antiproliferative drug effects, potentially leading to delayed healing of the endothelium and a higher risk of infection compared to BMS14. Additionally, the increased number of infections with DES may be attributed to their more widespread use as compared to BMS in PCIs.
Numerous studies have examined the incidence of bacteraemia after catheterisation procedures, reporting it in 0.1% of patients following cardiac catheterisation15 and in 0.6% after percutaneous transluminal coronary angioplasty (PTCA)16. Risk factors for bacteraemia after cardiac catheterisation include repeated catheterisation via the same vascular access, extended procedure duration (as in PTCA for chronic total occlusions), delayed removal of arterial sheaths, intra-aortic balloon pump (IABP) usage, poor haemostasis around the arterial sheath causing haematoma, and advanced age1617. Risk factors for stent infection identified in our case series included repeated use of the same local site, especially the femoral artery for puncture; reuse of hardware like balloons, wires, and catheters (common in resource-limited hospitals); multiple guidewire manipulations (e.g., interventions for chronic total occlusion); prolonged catheterisation exceeding 4 hours; inadequate sterile techniques; and diabetes mellitus (Table 4). Many catheterisation labs in our series were newly established and lacked regular microbiological surveillance. Inadequate aseptic precautions may have been due to insufficient training among newly appointed catheterisation laboratory staff. The majority of patients with stent infections in our series primarily presented with ACS ST-segment elevation myocardial infarction (ACS-STEMI). Some patients, such as manual labourers, might have had suboptimal hygiene and inadequate skin preparation, possibly due to the need for quick door-to-balloon times or deteriorating haemodynamics. In some cases, iodine-based skin preparation would not have been left for the recommended 3 minutes before puncture. Additionally, the application of surgical spirit after iodine may have diminished the iodine’s effectiveness. One stent infection occurred in a high-volume centre where over 250 angioplasties were performed monthly, including at least 150 primary angioplasties. Such high case volumes may unintentionally lead to lapses in aseptic precautions. It is worth noting that hardware reuse alone cannot account for the condition, as most reported stent abscess cases are from Western countries where hardware reuse is less common than in resource-limited developing nations.
Stent infections can either remain localised to the coronary artery and surrounding tissue or become more diffuse, involving multiple organ septic emboli18. In cases of multisystem involvement, mortality rates were high. However, except for 1 patient with renal impairment, none of our cases exhibited multisystem manifestations at presentation. Of the 11 stent abscess cases in our study, 6 were caused by Staphylococcus aureus (80%), 2 by Pseudomonas aeruginosa, and 3 yielded culture-negative results.
Stent infection symptoms usually emerge days to weeks after the initial coronary intervention. Although rare, delayed stent infections occurring 2-7 years after PCI have been reported1920. In our series, most patients developed fever within 2 weeks of the procedure. Notably, 1 patient presented with an abscess eroding through the diaphragm into the epigastrium 7 years after angioplasty.
A previously published smaller series21 reported fever and postinterventional angina as the most common presentations of coronary stent infections. Septicaemia developed at various times, from day 1 to 11 months after the procedure, with most cases occurring within the first month. Our patients presented with fever, pericarditis leading to effusion (sometimes causing tamponade), and postinterventional angina due to total occlusion of the stented segment. Our study, along with previous reports, suggests that patients who have undergone PTCA and present with angina and unexplained fever, particularly within 4 weeks of the intervention, should raise suspicion of stent-related infection and undergo an echocardiographic evaluation. A previously proposed diagnostic criterion for coronary artery stent infection emphasised the need for histopathological evidence in resected specimens or during autopsy for a definitive diagnosis, or fulfilling 3 out of 7 criteria for a possible diagnosis. Clinical presentations in our case series included febrile illness, pericarditis with effusion (sometimes causing tamponade), and acute stent thrombosis, and 1 case manifested as an epigastric swelling due to an abscess eroding through the diaphragm. While 2 of our patients presented with ACS, most presented with pericarditis, which led to effusion and tamponade in 4 cases. The pericardial fluid was haemorrhagic in most cases, and there was 1 instance of pyopericardium. None of our patients with recurrent ACS exhibited obvious prothrombotic tendencies.
Diagnosing coronary stent infection is challenging given its varied presentations22. Blood investigations revealing leucocytosis with a left shift, toxic granules, elevated CRP, and procalcitonin, along with positive blood cultures in cases of recent PTCA, should prompt suspicion of stent infection. Various imaging modalities, including TTE and TOE, CT, magnetic resonance imaging, and coronary angiography, are crucial for diagnosis. In some cases, a radio-labelled autologous leukocyte scan can localise the infection to the coronary arteries. Early stent infections may present with normal echocardiograms, with pseudoaneurysms developing later, potentially causing a delayed diagnosis if repeat TTE or CT scans are omitted, as observed in our series. Additionally, our study highlights the role of multimodal imaging, especially CT and PET-CT, in early infection detection. Pseudoaneurysm size can rapidly increase, as we noted in 2 cases, emphasising the importance of repeat imaging for assessing progression. A radio-labelled leukocyte scan could serve as an early confirmation or refutation of the diagnosis23. All our cases were diagnosed using conventional imaging techniques (mainly TTE/TOE), supported by laboratory infection evidence. TTE and TOE findings of stent abscesses were characterised by an echolucent cavity, ranging from 10 mm to 9 cm in size, with a well-defined thick wall, echogenic material inside, and a highly reflective linear stent shadow within the cavity.
Based on our extensive case series, the largest to date, we propose diagnostic criteria for stent abscess following stentocarditis:
Definitive diagnosis: confirmed during surgery or autopsy.
Probable diagnosis: a patient with a history of angioplasty, presenting with all 3 of the following, should be considered a probable stent infection case:
⢠Clinical presentation of febrile illness, pericarditis with effusion, or acute stent thrombosis
⢠Evidence of infection, such as blood culture positivity, organism isolation from pericardial fluid, or elevated procalcitonin in culture-negative cases
⢠TTE and/or TOE showing an echolucent cavity with an echogenic linear stent shadow or CT showing localised coronary dilation exceeding 1.5 times the diameter of the adjacent vessel, or PET-CT demonstrating focal increased uptake around the stent
The primary treatment for coronary stent infections is intravenous antibiotics targeting Staphylococcus aureus and Pseudomonas aeruginosa, often supplemented with surgery, which is necessary in most cases. We were able to successfully manage 3 cases medically with antibiotics targeting Staphylococcus and Pseudomonas for 6 weeks. Medically treated patients generally had smaller aneurysms, less than 20 mm. Despite antibiotic and/or surgical interventions, historically, mortality rates can exceed 50%.
Surgery for stent abscess is exceptionally challenging and longer in duration due to extensive dissection. In some cases, a considerable amount of pus needed drainage, with 1 case requiring presternotomy aspiration due to imminent rupture. Large abscesses were visible upon pericardial opening in some cases, while others revealed only unhealthy tissue. Tissue dissection was challenging, with severe friability complicating the process. Achieving haemostasis was difficult in some cases, requiring haemostatic fibrin glue and multiple large pledgetted 3-0 Prolene sutures (Ethicon). The infected pseudoaneurysm was opened, debrided, marsupialised, and the coronary artery stents were removed. In 1 case with multiple stents in the RCA, a single entire stent was retrieved, but only part of the second stent was able to be removed. Determining whether the entire stent was removed was often challenging, as the stent tends to deform and stretch during dissection. In most cases, distal vessels were grafted with a long saphenous vein. The necessity of resecting the infected coronary segment alongside complete stent removal remains an understudied aspect. As per 1 case report, 1 patient experienced new-onset myocardial dysfunction after surgery despite having a normal preoperative ejection fraction. Possible explanations include advanced sepsis and postoperative ischaemia resulting from coronary ligation with insufficient supply from the bypass graft to distal vessels2. None of our cases developed new-onset left ventricular dysfunction after surgery.
Given previous reports of ruptured left main stent pseudoaneurysms and the significant risk of residual infection leading to stent graft infection or in-stent restenosis when using covered stents, we did not consider this treatment modality for any of our patients24. We strongly discourage considering covered stents as a treatment option, as 1 of our patients developed sepsis despite complete surgical excision of the infected area and stent.
Table 4. Predisposing factors.
| Case | Puncture site | Same site repeat puncture within 1 week | Diabetes | Reuse of hardware | Average duration of procedure, minutes | Preceding fever/focus of infection | Approximate duration of sheath use, hours | Operator experience, years | Microbiology surveillance every 6 months |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Femoral | Yes | No | Yes | 45 | Yes | 5 | 8 | Yes |
| 2 | Femoral | No | No | Yes | 25 | No | 4 | 10 | No |
| 3 | Radial | No | No | NA | 25 | No | NA | >10 | NA |
| 4 | Femoral | No | Yes | Yes | 60 | No | 4 | >10 | No |
| 5 | Radial | No | No | NA | 45 | Yes | NA | 4 | NA |
| 6 | Femoral | Yes | Yes | NA | NA | No | 4 | 4 | No |
| 7 | Femoral | No | No | NA | 50 | Yes | NA | 6 | No |
| 8 | Femoral | Yes | No | Yes | 25 | No | 8 | >20 | Yes |
| 9 | Radial | No | Yes | Yes | 25 | No | 7 | 3 | No |
| 10 | Femoral | No | No | No | 40 | No | 6 | 3 | No |
| 11 | Radial | No | No | Yes | 30 | No | 4 | 3 | No |
| NA: not applicable | |||||||||
Conclusions
The infection of coronary stents following angioplasty, resulting in stent abscess formation, is an exceedingly rare clinical entity that demands a high level of clinical suspicion for accurate diagnosis. Prolonged febrile illness, pericarditis with pericardial effusion, recurrent ACS, and sepsis with multiorgan dysfunction following angioplasty should raise suspicion of this condition. The primary treatment approach involves prolonged antibiotic therapy aimed at Staphylococcus aureus and Pseudomonas aeruginosa, initiated promptly upon suspicion, combined with surgery in most cases. Preventative measures − including avoiding repeated groin punctures; stringent aseptic precautions in catheterisation labs; avoiding the reuse of catheters, guidewires, and balloons; and ensuring strict glycaemic control before interventions − can help minimise the incidence of stent abscess, a potentially deadly angioplasty complication with a high mortality rate.
Impact on daily practice
Percutaneous coronary interventions (PCIs) have become an integral part of managing coronary artery disease. The procedure has found global acceptance and is available to patients even in fairly remote areas of developing countries. It has emerged as a ubiquitous procedure and is carried out across the globe on a daily basis. Stent infection, as described in this article, is fortunately rare; however, it is devastating for the patient and the caregiver in case of its occurrence. Prolonged febrile illness, pericarditis with pericardial effusion, recurrent acute coronary syndrome, and sepsis with multiorgan dysfunction following angioplasty should raise suspicion of this condition. Literature on the management of such patients is sparse, and a management algorithm as described above will aid the cardiologists and physicians treating patients with febrile illness following PCI. Knowledge about the presentation of this deadly complication will aid its recognition and timely treatment.
Conflict of interest statement
The authors have no conflicts of interest to declare.

