Introduction
Excimer (excited dimer) laser ablates tissue using photochemical, photothermal and photokinetic mechanisms1. The first successful experience with excimer laser coronary atherectomy (ELCA) was reported in 19892. Subsequent randomised trials concluded that ELCA offered no additional benefit over balloon angioplasty and its usage became negligible34. More recently, the Excimer Laser LEsion Modification to Expand Non-dilatable sTents (ELLEMENT) trial demonstrated the feasibility of ELCA for the niche application of in-stent restenosis (ISR) in poorly expanded stents resistant to high-pressure balloon inflations often due to calcification5. This was a significant finding as stent underexpansion related to severe calcification remains an important cause of target lesion failure (TLF) and therapeutic options are limited67. There are limited further published data on ELCA usage for this purpose89101112.
Our institution elected to adopt ELCA in 2016 for the treatment of stents that remained underexpanded despite adequately sized very high-pressure balloon inflations. We report our initial experience in order to add to the literature with regard to the safety and efficacy of ELCA-assisted expansion of undilatable stents.
Methods
This was a retrospective observational study at a single tertiary referral hospital from March 2016 to February 2021.
Inclusion criteria were stent underexpansion of at least 30% minimum stent diameter (MSD) as compared to reference vessel diameter, confirmed by stent enhancement imaging, intravascular ultrasound (IVUS) or optical coherence tomography (OCT), as assessed by two operators. If the stent had been previously dilated and remained constrained at 26 atmospheres or greater, then further conventional treatment was not attempted and ELCA was the initial therapy. If the stent had never been subjected to 26 atmospheres, then a high-pressure inflation was performed and ELCA used only if the stent was constrained. De novo stents and stents with ISR were included.
Informed consent was obtained from all patients. The study was approved by the St Vincent’s Hospital Human Research Ethics Committee, Sydney, Australia – 2020/ETH01873.
Procedure
All procedures had at least two experienced percutaneous coronary intervention (PCI) operators with at least one, and usually both, having experience with ELCA. After early experience showed slow flow was common due to microbubble formation with significant electrocardiographic changes and chest pain, general anaesthesia was used frequently. Anaesthetists routinely attend the cardiac catheterisation laboratory for other complex procedures.
Stents that had not previously been subjected to very high pressures were first dilated at 26 atmospheres or greater and were not included unless they remained constrained. Stents known to be resistant to previous high-pressure balloon inflations were not required to be initially dilated. Thereafter, the stents were assessed angiographically for the degree of neointima. Where doubt existed, intracoronary imaging was used. If significant neointimal tissue was present within the stent, at least six trains of ELCA using saline flush were performed in an attempt to achieve tissue debulking. Thereafter, four to six trains were performed without flush or contrast such that only blood was in the ablative field in order to ablate tissue further. The laser remained in-stent at all times and was advanced both anterogradely and retrogradely at 0.5 to 1 mm per second.
The stent was then assessed angiographically and, if adequate tissue debulking had occurred, the technique progressed to seven contrast-enhanced (intermittent bolus) trains focused on the site of worst constraint. Constrained stents without significant in-stent neointimal tissue progressed immediately to this step. Slow flow was treated with intracoronary glyceryl trinitrate and adenosine (avoided in right coronary artery [RCA] lesions).
Following the above, the stent was then postdilated to at least 26 atmospheres with a non-compliant balloon at least 0.25 mm larger than the reference vessel diameter. Following successful ELCA, stent recoil was still observed. A slightly larger balloon resulted in a better final result and, in this small series, was not complicated by perforation, although the risk is acknowledged. If the stent could not be adequately expanded, then step three (contrast) was repeated. If the stent continued to be resistant, an ultra-high pressure inflation (36 atmospheres) was performed and, rarely, a larger ELCA catheter was used.
Finally, all lesions were treated with a drug-eluting balloon (DEB) after ELCA. Additional stents were not used in the target lesion.
Study endpoints and definitions
Device success was defined as the ability to cross the lesion with the ELCA catheter. Procedural success was defined as an increase of at least 50% MSD as measured by quantitative coronary angiography (QCA). Coronary angiograms were analysed offline using a validated system (Release 1.2.1; Philips Medical Systems) by an experienced radiologist. Adequate stent expansion was defined as MSD >70% of the reference vessel diameter and full stent expansion as MSD >90% of reference. MSD, reference vessel diameter and percent diameter stenosis were measured pre- and post-PCI. In the patients in whom intravascular imaging was used, the minimum lumen area (MLA) and minimum stent area (MSA) were measured pre- and post-PCI. The neointimal hyperplasia cross-sectional area was defined as MSA minus MLA. Calcium fracture was characterised by a break in peri-stent calcium. OCT images were acquired using the ILUMIEN OPTIS system (Abbott Vascular) and a 2.7 Fr OCT imaging catheter (Dragonfly OPTIS; Abbott Vascular). Offline analysis was performed using proprietary software (Abbott Vascular) by two experienced investigators.
The endpoints used to assess procedural safety were perforation, dissection in an uncovered segment of the target vessel, slow flow and periprocedural myocardial infarction (MI). During follow-up we also evaluated the occurrence of target lesion revascularisation (TLR), MI and death using the standardised definitions of the Academic Research Consortium13.
Statistical analysis
Statistical analysis was performed with SPSS, version 26 (IBM Corp.). Continuous variables are expressed as the mean±standard deviation (SD) and categorical variables as the frequency (%). Continuous variables were compared using Student’s t-test.
Results
Patient characteristics are summarised in Table 1. There were 24 patients with 31 lesions. Fifteen patients were referred from other centres. In two cases, the procedure was for a recently implanted stent: one with stent thrombosis and the other as prophylaxis. All other patients had recurrent restenosis partially due to poor stent expansion. The mean age was 71.9±9.6 years and 22 patients (92%) were male. Diabetes mellitus was present in 14 patients (50%), and 23 patients (96%) had a history of hypertension and dyslipidaemia. Fourteen patients were current or ex-smokers. Eight patients (33%) had undergone prior coronary artery bypass grafting (CABG). Twenty patients presented with stable angina and four with acute coronary syndrome. All patients had significant symptoms and no other option for treatment.
The index stent was a drug-eluting stent (DES) in 16 lesions, of which three were a first-generation DES (Table 2). Six lesions had been treated with a bare metal stent at the time of the index procedure. The mean time since implantation was 13.5±21.9 years. The mean number of prior procedures for ISR was three (range one to seven) and 14 lesions had multiple layers of stent.

The target vessel was native in 29 lesions and was a saphenous vein graft (SVG) in two lesions (Table 3). There were no aorto-ostial lesions. The American College of Cardiology/American Heart Association lesion type was B2 or C in 18 lesions (58%) and all lesions were at least moderately calcified (Figure 1). The mean lesion length was 19.2±12.2 mm.

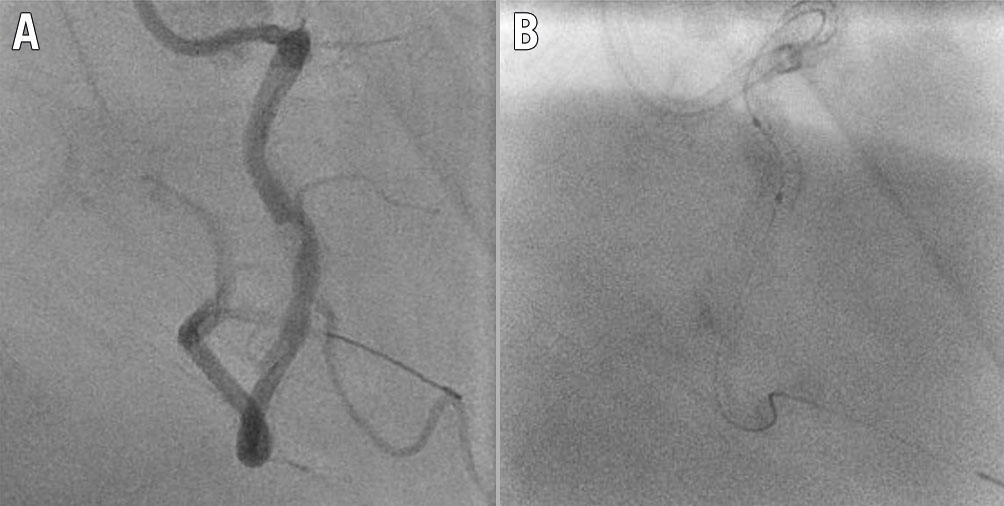
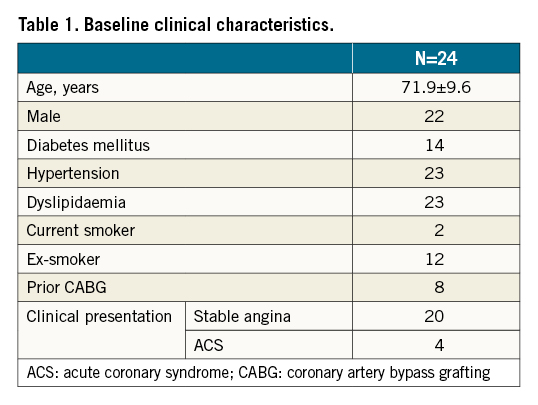
Figure 1. Lesion 15. Pre-percutaneous coronary intervention angiogram (A) and stent enhancement imaging (B) showing stent underexpansion due to severe peri-stent calcification outside the stent in the mid-right coronary artery.
Device and procedural success were achieved in all 31 lesions. The mean MSD increased from 0.82±0.59 mm to 2.62±0.72 mm, as measured by QCA (acute gain 1.81±0.62 mm, p<0.001). Adequate stent expansion was achieved in all lesions, of which full stent expansion was achieved in 17 (Figure 2).

Figure 2. Stent expansion. Adequate: minimum stent diameter >70% of reference vessel diameter; Full: minimum stent diameter >90% of reference vessel diameter; LAD: left anterior descending; LCx: left circumflex; PCI: percutaneous coronary intervention
In the three patients for whom OCT data were available pre- and post-PCI, the mean MLA increased from 2.75±0.88 mm2 to 5.49±1.23 mm2 due to an increase in the mean MSA from 3.73±0.58 mm2 to 6.38±0.77mm2 and a decrease in the mean neointimal hyperplasia cross-sectional area from 0.98±0.31 mm2 to 0.89±0.77 mm2 (Table 4). Adequate stent expansion and multiple peri-stent calcium fractures were observed post-PCI (Figure 3, Figure 4).

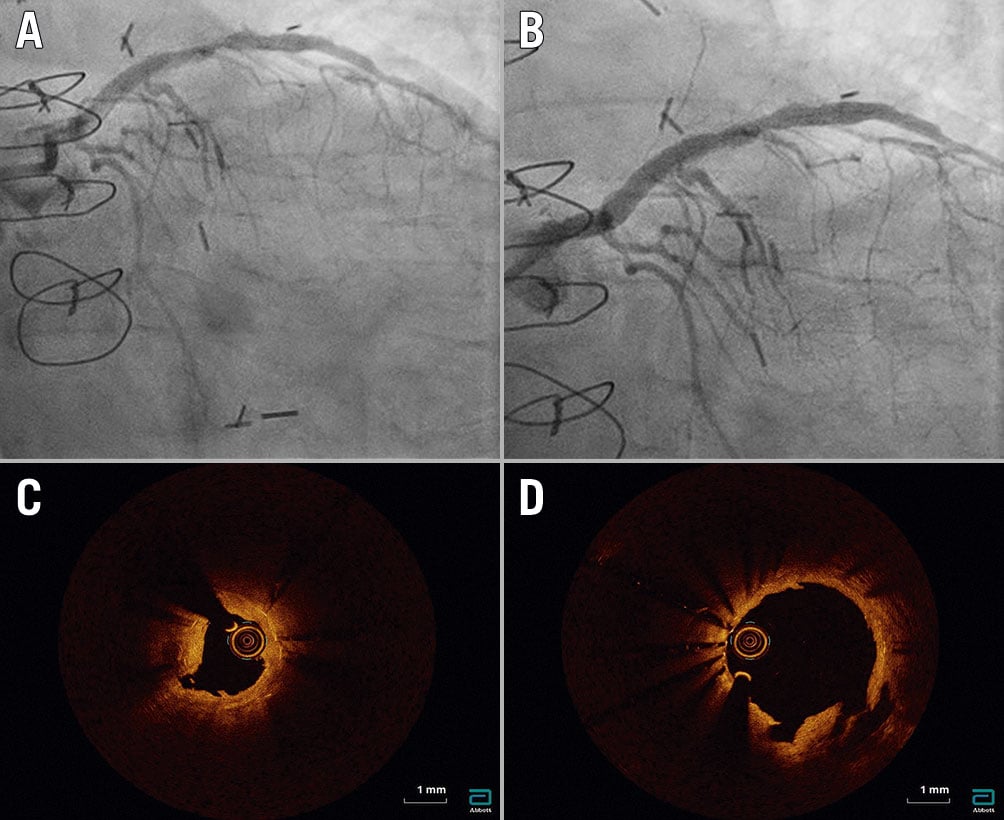
Figure 3. Lesion 23. A) Pre-percutaneous coronary intervention (PCI) angiogram showing severe in-stent restenosis (ISR) in the left anterior descending artery. B) Post-PCI angiogram showing mild residual stenosis. C) Pre-PCI optical coherence tomography (OCT) showing ISR and stent underexpansion (minimum stent area [MSA] 3.42 mm2) due to peri-stent calcium. D) Post-PCI OCT showing adequate stent expansion (MSA 5.62 mm2), a reduction in neointimal tissue and multiple peri-stent calcium fractures.
Figure 4. Lesion 29. A) Pre-percutaneous coronary intervention (PCI) angiogram showing severe in-stent restenosis in the proximal left anterior descending artery. B) Post-PCI angiogram showing full stent expansion. C) Pre-PCI optical coherence tomography (OCT) showing two layers of underexpanded stents (minimum stent area [MSA] 3.38 mm2) due to thick peri-stent calcification despite recent use of intravascular lithotripsy and high-pressure inflations. D) Post-PCI OCT showing full stent expansion (MSA 7.14 mm2) and a reduction in neointimal hyperplasia tissue.
The mean follow-up period was 21.3±16.3 months. At six-month follow-up (N=26 lesions), there were nine major adverse cardiac events (MACE); six periprocedural MIs due to slow flow and/or transient ST-segment elevation; two cardiac deaths; and one TLR (Table 5). There were no perforations or dissections in uncovered segments of the target vessel.
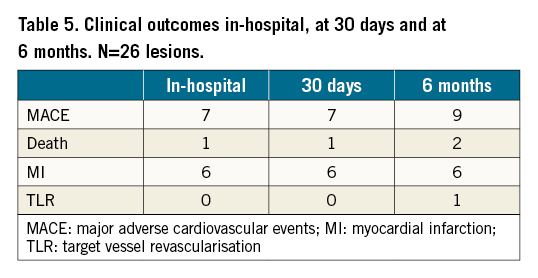
There was one in-hospital cardiac death and two further cardiac deaths at five-month and 33-month follow-up. TLR occurred in five patients: one at three months which was treated with a DEB; three at nine months which were treated with rotablation and DEB; and one at 23 months which was successfully treated with ELCA.
Discussion
ELCA ablates tissue in three ways: photochemical (fracture of molecular bonds using ultraviolet light pulses with a shallow penetration depth <0.1 mm from the catheter tip), photothermal (tissue vaporisation), and photokinetic mechanisms (rapidly expanding and collapsing vapour bubbles generate pressure pulses which further ablate deeper intimal tissue and medial calcification)1. The flush and bathe technique was designed by Tcheng et al in the 1990s, to replace blood and contrast in the ablative field with saline, in order to attenuate the generation of detrimental pressure pulses that contribute to dissection14. Interestingly, a high-energy contrast flush technique was subsequently developed specifically for underexpanded stents in heavily calcified lesions which fail to fully expand with the conventional flush and bathe technique8. Powerful pressure pulses >100 atmospheres are used to enhance the photomechanical effect of ELCA while the layer of stent protects the vessel wall.
Our initial experience at a centre with very experienced operators demonstrated that in highly selected patients with undilatable stents and recurrent restenosis, contrast-enhanced ELCA effectively facilitated stent expansion when no other treatment option was available. This led to larger final lumen and stent dimensions. To the best of our knowledge, only one other study has validated the safety and efficacy of ELCA in a similar group of patients with recurrent ISR in the setting of underexpanded, undilatable stents10. The ELLEMENT registry was a small pilot study which evaluated the feasibility of ELCA with contrast flush to treat 25 patients with undilatable stents with ISR when high-pressure non-compliant balloon angioplasty was ineffective5. Procedural success, defined as a 20% increase in MSD as measured by QCA, or an increase of least 1 mm2 for MSA as measured by IVUS, was achieved in 96.4% of patients (acute gain in MSD 1.1±0.7 mm, p<0.001, and acute gain in MSA 3.7±1.6 mm2, p<0.001). Slow flow or transient ST-elevation occurred in two patients. There was one cardiac death and one TLR at six-month follow-up.
A distinctive feature of our cohort is that fourteen lesions (45%) had multiple layers of stent, up to five layers deep, and indeed our study is the first to demonstrate the success of ELCA in this particularly challenging setting. Another novel aspect of our study is the use of DEBs to prevent neointimal hyperplasia without an additional layer of DES, which has been shown to be associated with new stent underexpansion and a higher prevalence of long-term MI and TLR, especially in the setting of old stent underexpansion and peri-stent calcium15. To date, there has been only one study evaluating the outcomes of patients with ISR treated with ELCA plus DEB angioplasty16. However, the lesion complexity was not described in detail. In particular, the prevalence of stent underexpansion and peri-stent calcium was not reported.
Intravascular imaging offers invaluable insights into the mechanisms of ELCA and may help to maximise debulking and optimise outcomes. Using OCT, we observed that stent expansion was achieved by two mechanisms: 1) ablation of neointimal tissue, and 2) peri-stent calcium fractures, although the mechanistic implications are speculative, as intracoronary imaging was used in a minority of patients. The seminal study which determined the mechanisms of ELCA for the treatment of ISR using IVUS showed that lumen enlargement was due to a combination of tissue ablation during ELCA causing a reduction in neointimal hyperplasia volume plus tissue extrusion and additional stent expansion during adjunctive balloon angioplasty17. Using OCT, ELCA has since been shown mechanistically to facilitate fracturing of thick, napkin-ring peri-stent calcium9. A recent study using OCT to evaluate the effectiveness of ELCA plus PCI or PCI alone to treat patients with underexpanded stents and ISR with peri-stent calcium found that the MLA after intervention was significantly larger in the ELCA group compared to the non-ELCA group due to more peri-stent calcium fractures which facilitated a larger gain in MSA10. Importantly, ELCA led to more calcium fractures in more severely calcified lesions with greater calcium thickness. In our cohort, we were able to achieve adequate stent expansion in all lesions despite moderate or severe calcification. This is a significant finding as stent underexpansion related to more severe calcification remains an important cause of TLF and therapeutic options are limited.
Although the use of ELCA is currently limited to niche applications, this technology is poised to become an indispensable tool in the armamentarium for optimising outcomes in complex PCI in the future. IVUS studies have shown that stent underexpansion is the main mechanism of restenosis in the DES era18. Other therapeutic options are limited. A handful of case studies have demonstrated the feasibility of novel coronary intravascular lithotripsy (IVL) technology in facilitating stent expansion by fracturing severely calcified lesions using acoustic waves19202122232425262728. However, larger trials with longer-term follow-up are needed to determine the safety and efficacy of coronary IVL in this challenging setting. As a case in point, one patient in our cohort was referred with severe in-stent restenosis in two layers of underexpanded stents due to thick peri-stent calcification in the proximal left anterior descending (LAD) artery despite recent use of coronary IVL and high-pressure inflations (Figure 4). We were able to achieve full stent expansion with ELCA. A limitation of the coronary IVL system is a higher crossing profile and lower deliverability compared with ELCA, which demonstrated 100% deliverability both in our study and in the ELLEMENT registry5. Another limitation of the coronary IVL system is a higher cost compared to ELCA. ELCA has Therapeutic Goods Administration approval for use in Australia whereas IVL must be accessed through the Special Access Scheme.
Importantly, in our cohort, there were no perforations or procedural deaths. Slow flow was relatively common and is, in our opinion, a significant limitation of ELCA. It is related to contrast usage and the number of trains. This led to the adoption of frequent general anaesthesia. However, more recently, we have moved back to conventional sedation but are waiting for longer periods between the repeated applications of laser and we have observed markedly reduced slow flow.
At a mean follow up of 21.3 months, TLR occurred in five lesions, which is acceptable given that the bulk of the patients were plagued by recurrent symptomatic restenosis and no new stents were deployed. However, there were three cardiac deaths which are worthy of discussion. The first patient was referred for ELCA after a non-ST-elevation myocardial infarction (NSTEMI) with severe left ventricular dysfunction. He was graft-dependant with severe ISR in a SVG to the left circumflex (LCx) artery which was undilatable. He also had critical calcified long-segment disease in his LAD beyond the graft. The SVG was successfully treated, however, whilst in hospital he had further episodes of pain with electrocardiographic changes and troponin elevation. The SVG was reimaged with OCT and was widely patent with no thrombus and, as the LAD was considered very high risk, a trial of ongoing medical therapy was attempted, complicated by recurrent anterior NSTEMI and then a fatal bleeding complication. It was not considered that ELCA was the direct cause of death, rather the untreated residual disease. The second patient similarly had severe left ventricular dysfunction, renal impairment, peripheral vascular disease with amputations and had recurrent restenosis of his LAD, LCx and RCA. The LAD and LCx were successfully treated with ELCA but the RCA seemed adequately expanded without ELCA, so was not treated. Five months later he presented with an inferior NSTEMI, complicated by multiorgan failure and was palliated. His death was considered unlikely to be related to ELCA. The final patient had previously presented with an aborted ST-segment elevation myocardial infarction (STEMI) due to a poorly expanded stent in his RCA and a pristine angiographic result was achieved with ELCA with full stent expansion in a large RCA. Despite having been well for 33 months, he had sudden cardiac death at home. He had known left ventricular dysfunction and previous CABG. In summary, the three deaths cannot be ignored but ELCA did not seem to be directly related. Rather, the patients were part of a very high-risk group.
Limitations
This was a single-centre retrospective study. It is important to stress that this was a niche experience at a centre with operators experienced with ELCA. A feature of our experience was our selection criteria, which limited inclusion to the type of patient with known underexpanded stents despite adequately sized very high-pressure inflations or recurrent restenosis in whom no other treatment option was available.
Intravascular imaging was performed in five patients, but imaging data pre- and post-PCI was only available for three patients.
Conclusions
ELCA successfully facilitated stent expansion with good short- and long-term results at a centre with very experienced operators in highly selected patients with undilatable stents and recurrent restenosis when no other treatment option was available. This was achieved at the cost of relatively frequent slow flow.
Impact on daily practice
Undilatable stents with severe peri-stent calcification are an important cause of target lesion failure and therapeutic options are limited. Our niche experience at a centre with experienced operators demonstrated that excimer laser coronary atherectomy led to larger final lumen and stent dimensions in highly selected patients with undilatable stents and recurrent restenosis at the cost of relatively frequent slow flow.
Conflict of interest statement
The authors have no conflicts of interest to declare.
