Introduction
Transcatheter aortic valve implantation (TAVI) is a revolutionary procedure that has expanded the therapeutic opportunities to most patients, becoming an increasingly preferred method for severe aortic stenosis (AS), not only for the elderly but also expanding to younger populations as data emerge, subject to the decisions of the Heart Team1. Such expansion of treatment to a wider population is increasing the financial burden of healthcare, product availability, and affordability. Therefore, cost-effective and reliable alternatives are necessary to improve outcomes worldwide. The Crea Aortic Valve (CAV [Onecrea Medical, Inc]) is designed to increase product availability at an affordable cost without compromising quality.
The aim of the study was to assess the technical usability, device performance, and safety of Onecrea Medical’s CAV at 30 days and 6 months for the first time in humans.
Methods
Study design
This was a prospective, phase 1 study performed in three high-risk patients with severe symptomatic AS at a single centre. The approval of the ethics committee of the hospital and informed consent from all patients were obtained prior to enrolment. Adherence to the principles of Good Clinical Practice and compliance with the Declaration of Helsinki were ensured2. The principal investigator (PI) attested to the accuracy and completeness of the study and the data published. The study was sponsored by Onecrea Medical, who provided the valves and technical support for TAVI. However, the PI and the authors had the liberty to inspect and interpret the data for analysis and manuscript preparation. The STROBE checklist for observational studies was adhered to while preparing the manuscript.
Patients
The patients included in the study had severe native calcific aortic stenosis (aortic valve [AV] area ≤0.8 cm2, a mean AV gradient of >40 mmHg, or a maximum aortic jet velocity [Vmax] of >4.0 m/s), were classified as having a small native aortic annulus (<23 mm in diameter)3 and were judged by a Heart Team, including a cardiac surgeon, to be at high risk or inoperable for open heart surgery. The Society of Thoracic Surgeons (STS) scores for the three patients were 4.80%, 7.95%, and 4.96%4. Although the inclusion criteria initially were stated as STS ≥8%, the PI had the final say in the patient selection, based on other criteria such as age, frailty, etc.
Inclusion criteria were as follows: age ≥18 years; informed consent given for procedure and follow-up; life expectancy of ≥1 year; symptomatic severe AS with high risk for open surgery; vascular and aortic arch anatomy suitable for TAVI as per the instructions for use and the PI’s judgement; and aortic arch >80 mm (considering the arch is an arc of the circle, the diameter of the circle formed by the arc) or with such an anatomy that a 100 mm shaft should be able to fit in a straight line at the aortic arch.
Exclusion criteria were as follows: mechanical or biological prosthesis in position at the AV; known hypersensitivity or contraindication to aspirin, heparin, clopidogrel, nitinol (titanium or nickel) or any device component, or sensitivity to contrast media, which could not be adequately premedicated; ongoing sepsis, including active endocarditis; bicuspid AV; pregnancy; and left ventricular ejection fraction (LVEF) <30%.
Medications
The patients were administered medications as per the hospital standard of care for TAVI and catheterisation procedures. Intravenous heparin was administered during the implant procedure, with a recommended target activated clotting time of 250 seconds. After the TAVI procedure, lifelong aspirin 81 mg daily in combination was recommended in addition to previously administered concomitant medications. Patient 1 and patient 2 were already receiving clopidogrel 75 mg and ticagrelor 90 mg, respectively. Patient 3, who had an indication for oral anticoagulation, paused this oral anticoagulant therapy in the periprocedural period but resumed it postprocedurally when there were no bleeding complications. Other postprocedural medications were administered based on hospital-specific procedures and patient indications.
The device
The Crea Aortic Valve System consists of 3 components: the transcatheter aortic valve, the delivery system (catheter), and the compression loading system (CLS) (Figure 1, Figure 2, Figure 3). The valve is manufactured by suturing valve leaflets and a skirt, made from bovine and porcine pericardium, respectively, onto a self-expanding, radiopaque nitinol stent. The valve tissues are processed to resist calcification. The transcatheter aortic valve is self-expanding, self-centring, and designed to be supra-annular when placed in the aortic annulus. The valve is delivered through an 18 Fr delivery system via the femoral artery. The CAV includes the following features (technology): (1) concave and convex shape to fit the anatomy of the aorta, which leads to self-anchoring in the optimal position. The concave shape of the stent helps in the anchoring of the CAV at the annulus. The convex shape of the stent sits in the sinus of Valsalva and helps seal the device in position. 2) Dual-cell configuration: dense cells at the base to provide the necessary radial force and open cells at the top to favour coronary access and prevent coronary occlusion. 3) Differential radial force: higher radial force at the base anchor at the annulus and lower radial force at the top to prevent trauma to the aorta. 4) Commissure design: leaflet attachments designed to ensure smooth commissure and high durability. The commissure also contains a marker for valve deployment and potential commissure alignment. 5) Optimised effective orifice area (EOA; ~1.9 mm2, ~2.5 mm2, and ~2.7 mm2 for CAV sizes 23 mm, 26 mm, and 29 mm, respectively). 6) Outer skirt design to prevent paravalvular leak (PVL). 7) The valve is implanted ~2-4 mm below the annulus, and implantation depth is calculated using the band formed by the crimped nitinol frame. The Crea Aortic Valve uses an 18 Fr delivery system and 18 Fr introducer sheath. The delivery system has a 14 Fr inline sheath to use in patients with a narrow femoral artery that cannot accommodate an 18 Fr introducer sheath.
Extensive bench testing and animal studies were performed on the CAV to ensure the safety and efficacy of the device: (1) biocompatibility tests demonstrated that the CAV is highly biocompatible. 2) Anticalcification studies demonstrated that the valve treatment process provides approximately a 90% reduction in calcification. 3) Hydrodynamic tests showed that the valve meets the ISO 5840 requirements. 4) Haemodynamic fatigue tests were performed to ensure that the device was able to perform >200 million cycles. 5) Many acute animal studies were performed over the course of the device’s development. Chronic animal studies were successfully performed in 4 animals for >140 days. These bench and animal studies demonstrated that the device was safe and functioned as intended (Table 1, Table 2).
The valve is available in three sizes, to be chosen according to the aortic annulus diameter (Table 3, Figure 1). The valve is recapturable and repositionable. The delivery system handle has a rotating knob to enable precise positioning of the implant at the aortic annulus. The delivery system is compatible with a 0.035” (0.889 mm) guidewire. The distal (deployment) end of the system features an atraumatic, radiopaque tip and a capsule that covers and maintains the valve in a crimped position. The delivery system uses a single spine shaft to increase flexibility and ease of crossing the aortic arch. The handle is located on the proximal end of the delivery system and is used to load and deploy the valve (Figure 2). The handle also has a quick-release button which compresses and slides for unsheathing and implantation of the device without using multiple rotations of the rotating knob. The CLS compresses the valve and aids in loading the valve into the delivery system, and it consists of (i) a polytetrafluoroethylene tube (straight tube), (ii) an assist cone, (iii) an eyelet cone, (iv) a loading ring, and (v) a sealing ring cone (Figure 3).
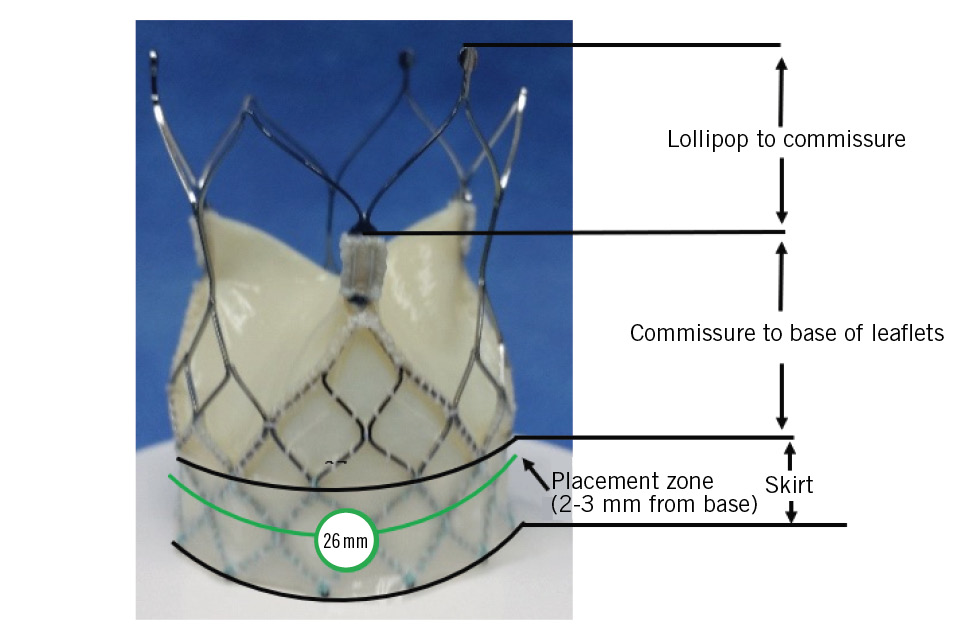
Figure 1. Crea Aortic Valve size 26 mm.

Figure 2. Delivery system.
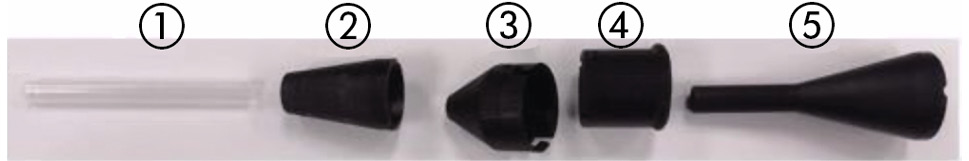
Figure 3. The components of the compression loading system. 1) Polytetrafluoroethylene tube (straight tube). 2) Assist cone. 3) Eyelet cone. 4) Loading ring. 5) Sealing ring cone.
Table 1. Accelerated wear testing with hydrodynamic testing at 0, 50, 100, 150, and 200 million cycles (bench tests).
| Samples | Before fatigue | After 50M cycles | After 100M cycles | After 150M cycles | After 200M cycles | Pass/fail | ISO req* | |
|---|---|---|---|---|---|---|---|---|
| 1906001-HV | EOA, cm2 | 2.47 | 2.14 | 2.06 | 2.14 | 2.09 | Pass | ≥1.70 |
| Regurgitant vol, % | 9.15 | 11.96 | 12.91 | 22.21 | 6.27 | Pass | ≤25% | |
| 1906002-HV | EOA, cm2 | 2.38 | 2.05 | 2.07 | 2.06 | 2.03 | Pass | ≥1.70 |
| Regurgitant vol, % | 4.87 | 6.47 | 8.46 | 9.02 | 6.94 | Pass | ≤25% | |
| 1907002-HV | EOA, cm2 | 2.09 | 2.09 | 2.08 | 2.14 | 2.05 | Pass | ≥1.70 |
| Regurgitant vol, % | 7.67 | 16.45 | 17.34 | 5.68 | 3.21 | Pass | ≤25% | |
| *ISO 5840 requirements. EOA: effective orifice area | ||||||||
Table 2. Epicardial echocardiography data at implant and term (animal tests).
| Study ID | Timepoint | Peak pressure gradient, mmHg | Mean pressure gradient, mmHg | Cardiac output, L/min | EOA, cm2 | Heart rate,per min | Total aortic regurgitation |
|---|---|---|---|---|---|---|---|
| TKB-3 | Implant | 3 | 2 | 5.4 | 3.7 | 78 | Trace |
| Term | 8 | 5 | 2.7 | 1.34 | 68 | None | |
| TKB-4 | Implant | 5 | 3 | 6 | 3.6 | 75 | None |
| Term | 2 | 2 | 2.7 | 2.77 | 53 | None | |
| TKB-5 | Implant | 30 | 14 | 7.5 | 1.59 | 106 | – |
| Term | 15 | 9 | 3.7 | 1.28 | 110 | None | |
| TKB-7 | Implant | 12 | 5 | 4.6 | 1.83 | 84 | None |
| Term | 7 | 4 | 5.5 | 3.48 | 95 | None | |
| EOA: effective orifice area | |||||||
Table 3. Crea Aortic Valve TAVI size reference guide.
| TAVI model | Size | Aortic annulus diameter |
|---|---|---|
| CAVS-23 | 23 mm | 17 mm-20 mm |
| CAVS-26 | 26 mm | 20 mm-23 mm |
| CAVS-29 | 29 mm | 23 mm-26 mm |
| CAVS: Crea Aortic Valve System; TAVI: transcatheter aortic valve implantation | ||
The procedure
1. Preprocedural evaluation: All patients underwent a preprocedural computed tomography (CT) angiography (CTA) for measurements around the AV. A transthoracic echocardiographic (TTE) assessment was done to measure the native valve area, the Vmax, the mean (MG) and peak (PG) pressure gradients, pulmonary artery systolic pressures, and LVEF.
2. TAVI was performed through a percutaneous transfemoral approach in patients 2 and 3. Patient 1 also underwent a transfemoral approach but required a femoral surgical cutdown due to a previous aortobifemoral bypass. The valve was released under rapid pacing in all three patients. The valve was placed with the skirt within the aortic annulus (approximately 4 mm to 6 mm below the virtual basal ring).
Follow-up
Any complaints of chest pain, dyspnoea, or palpitations were recorded and medications were reviewed during the hospital stay, at 30 days, and at 6 months, with further follow-up planned at 1 year. TTE was performed soon after the procedure, before discharge, and at the 1- and 6-month clinical follow-ups to monitor the valve area, Vmax, MG, PG, and PVL.
Outcome measures
The updated composite endpoints post-TAVI as described by the Valve Academic Research Consortium (VARC)-3 were assessed in the three patients as follows: (1) technical success (at exit from procedure room); (2) device success (at 30 days); (3) early safety (at 30 days); (4) clinical efficacy (at 6 months); (5) usability and ease of operation of the delivery system5.
Case 1
Preprocedural evaluation: an 82-year-old female was referred for TAVI due to severe symptomatic AS diagnosed during a preoperative evaluation for the treatment of a giant infrarenal abdominal aortic aneurysm (AAA). She had New York Heart Association (NYHA) Class II symptoms, was frail (44 kg and 158 cm), and was a heavy smoker at the time of presentation. Physical examination findings included an aortic ejection murmur grade 2-3, a pulsatile abdominal mass (the AAA, confirmed on ultrasound), and diminished tibial pulses (1+). Her electrocardiogram (ECG) showed sinus rhythm, a PR interval of 0.20 ms, and a QRS duration of 0.06 ms. She was stratified as being at high risk, with a European System for Cardiac Operative Risk Evaluation (EuroSCORE) II 8.28%6. TTE showed a valve area of 0.7 cm2 and an MG of 45 mmHg, and the CTA revealed a porcelain aorta (Table 4, Table 5, Figure 4A–Figure 4B).
Procedure: the patient underwent staged procedures under general anaesthesia with a temporary pacemaker. Stage 1: (a) coronary angiography (CA) showed severe left main coronary artery stenosis, (b) aortic valvuloplasty (18×40 mm Valver balloon [Balton]), (c) open AAA repair with an aortobifemoral graft using an 8 mm femoral conduit. Stage 2: (a) percutaneous coronary intervention (PCI) with a stent into the left main coronary artery, (b) TAVI with Onecrea Medical’s 26 mm valve under rapid pacing at a depth of 4-6 mm in the left ventricular outflow tract (LVOT) and a final MG of 12.8 mmHg. The device was implanted using the cusp-overlap technique.
Table 4. Patient demographics.
| Parameters | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Age, years | 82 | 88 | 87 |
| Sex | Female | Female | Female |
| Height, cm | 158 | 156 | 153 |
| Weight, kg | 44 | 55 | 75 |
| NYHA Class | II | III | II |
| EuroSCORE II | 8.28 | 12.94 | 3.41 |
| STS mortality score, % | 4.80 | 7.95 | 4.96 |
| Frailty16 | Yes | Yes | Yes |
| COPD/chronic lung disease | Yes | Yes | No |
| CKD | No | Yes | No |
| Hypertension | No | No | No |
| Diabetes | No | Yes | No |
| Hypothyroidism | No | Yes | No |
| S. Creatinine, mg/dL | 0.7 | 1.81 | 0.94 |
| Permanent pacemaker | No | No | Yes |
| Stroke | No | No | No |
| Prior CABG | No | No | No |
| Prior PTCA | Yes | Yes | No |
| Previous MI | No | Yes | No |
| CABG: coronary artery bypass graft; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; EuroSCORE: European System for Cardiac Operative Risk Evaluation; MI: myocardial infarction; NYHA: New York Heart Association; PTCA: percutaneous transluminal coronary angioplasty; STS: Society of Thoracic Surgeons | |||
Table 5. Transthoracic echocardiography characteristics.
| Patient 1: G-V-01-01 | ||||
|---|---|---|---|---|
| Data | Pretreatment | Post-TAVI | 3 weeks | 6 months |
| Vmax, m/s | 4.33 | 2.39 | 2 | 1.7 |
| MG, mmHg | 45 | 12.8 | 8 | 5 |
| PG, mmHg | 75 | 22.8 | 18 | 12 |
| AVA, cm2 | 0.7 | 1.7 | 1.6 | 1.8 |
| PAP, mmHg | 21 | 20 | 20 | 20 |
| LVEF, % | 75 | 66 | 75 | 75 |
| PVL | Trivial | Trivial | No PVL | |
| Patient 2: MDB-01-02 | ||||
| Data | Pretreatment | Post-TAVI | 1 month | 6 months |
| Vmax, m/s | 3.8 | 1.9 | 2 | 1.3 |
| MG, mmHg | 33 | 8 | 9 | 3 |
| PG, mmHg | 57 | 15 | 19 | 9 |
| AVA, cm2 | 0.5 | 1.5 | 1.55 | 1.8 |
| PAP, mmHg | 50 | 40 | 30 | 30 |
| LVEF, % | 55 | 60 | 60 | 60 |
| PVL | No PVL | No PVL | No PVL | |
| Patient 3: E-F 01-03 | ||||
| Data | Pretreatment | Post-TAVI | 4 weeks | 6 months |
| Vmax, m/s | 4.2 | 1.3 | 1.5 | 1.9 |
| MG, mmHg | 42 | 4 | 6 | 6 |
| PG, mmHg | 68 | 7 | 9 | 9 |
| AVA, cm2 | 0.7 | 1.4 | 1.7 | 2.0 |
| PAP, mmHg | 20 | 40 | 30 | 30 |
| LVEF, % | 60 | 60 | 60 | 65 |
| PVL | Trivial | No PVL | No PVL | |
| AVA: aortic valve area; LVEF: left ventricular ejection fraction; MG: mean pressure gradient; PAP: pulmonary artery pressure; PG: peak pressure gradient; PVL: paravalvular leak; TAVI: transcatheter aortic valve implantation; Vmax: maximum velocity | ||||
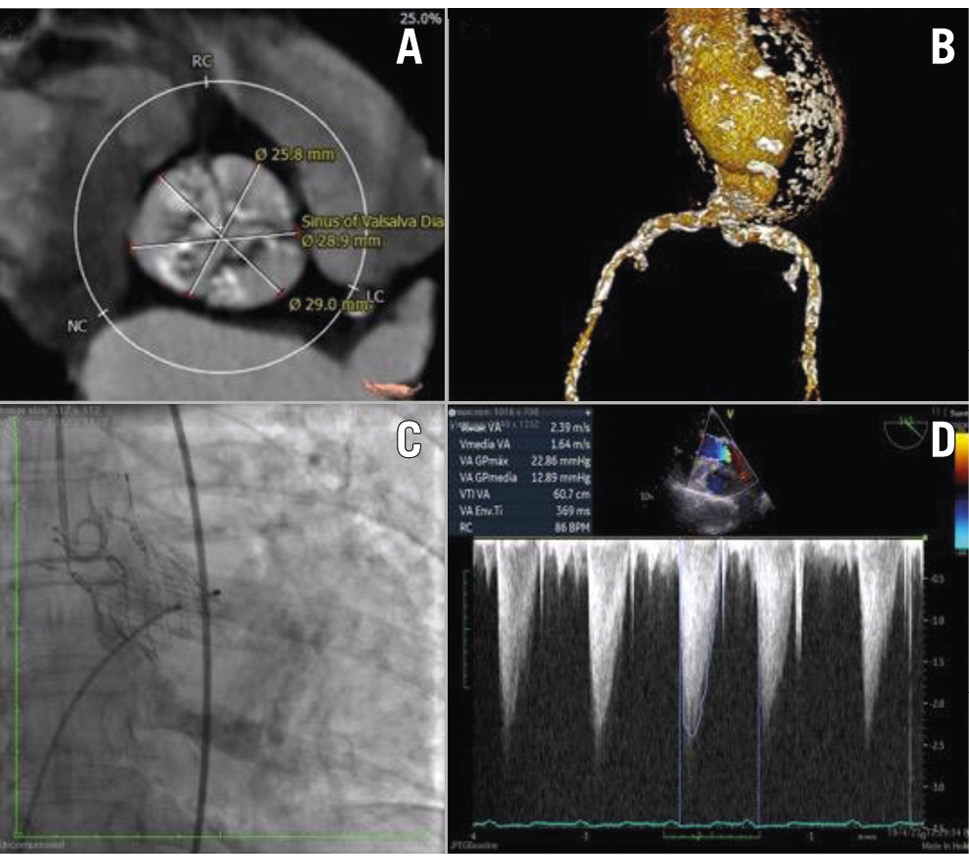
Figure 4. Essential periprocedural imaging. (A,B) CT angiography of aortic annulus, abdominal aortic aneurysm, and calcified access (patient 1); (C) periprocedural aortogram and (D) procedural transoesophageal echocardiography post-TAVI (patient 2). CT: computed tomography; TAVI: transcatheter aortic valve implantation
Case 2
Preprocedural evaluation: an 88-year-old female with diabetes, hypothyroidism, hypercholesterolaemia, and chronic kidney disease with severe symptomatic AS (NYHA III). She had previously been admitted to the emergency room experiencing heart failure that required mechanical ventilation, emergency balloon aortic valvuloplasty (BAV; 20×40 mm balloon) and PCI with stenting of the left main coronary artery for severe ostial stenosis. On physical examination, she weighed 55 kg and was 156 cm tall. There was aortic ejection murmur grade II/IV on auscultation and poor lower limb pulses (tibial pulses: left: 1+, right: 0). The ECG showed sinus rhythm, PR interval of 0.20 ms and QRS duration of 0.06 ms. Her EuroSCORE II was 12.94%. Table 6 reports the CT scan analysis.
Procedure: the patient underwent TAVI via the transfemoral route with a 26 mm Onecrea Medical valve under local anaesthesia and conscious sedation. The valve was released at a depth of 4-6 mm in the LVOT under rapid pacing, using a coplanar view. The left main PCI and BAV were performed on 23 March 2023, and the TAVI was carried out on 17 May 2023 (Figure 4C–Figure 4D). Post-dilatation was performed with a 23×40 mm Valver balloon to correct moderate paravalvular leak. A mild residual PVL was caused by severe calcification of the native aortic valve.
Table 6. Preprocedural CTA characteristics.
| Parameters | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Annulus perimeter, mm | 66.8 | 68.6 | 65.7 |
| Annulus maximum diameter, mm | 22.3 | 18 | 22.6 |
| LVOT perimeter, mm | 65.2 | 70 | 62.5 |
| SoV diameter, mm | 28.9 | 26.6 | 27.9 |
| STJ diameter, mm | 23.3 | 28.2 | 26.5 |
| AV characteristics | Porcelain aorta,moderate valve calcification | Severe symmetriccalcification of cusps | Severe annularand LVOT calcification |
| Left coronary height, mm | 19.5 | 16.9 | 9.8 |
| Right coronary height, mm | 16.2 | 10.7 | 19.7 |
| Femoral access | Calcification and tortuosity | Calcification and tortuosity | Calcification and mild tortuosity |
| AV: aortic valve; CTA: computed tomography angiography; LVOT: left ventricular outflow tract; SoV: sinus of Valsalva; STJ: sinotubular junction | |||
Case 3
Preprocedural evaluation: an 87-year-old female patient with severe AS and hypertension presented with shortness of breath (NYHA II). She was on a permanent pacemaker due to experiencing an advanced atrioventricular block (AVB) in 2013. Paroxysmal atrial fibrillation was detected in 2015, and therefore, she was on rivaroxaban. A systolic aortic murmur was present, and femoral and dorsalis pedis pulses were present (3+), with no oedema. Her EuroSCORE II was 3.41%, suggestive of a moderate surgical risk. The preprocedural TTE showed severe AS, mild aortic regurgitation, Vmax of 4.2 m/s, MG of 42 mmHg, PG of 68 mmHg, AV area of 0.7 cm2, and a left ventricular ejection fraction of 60% (Table 5). The CTA findings are disclosed in Table 6.
Procedure: the patient underwent a percutaneous transfemoral TAVI under local anaesthesia with conscious sedation. The preprocedural coronary angiography showed no significant coronary artery disease. Valvuloplasty was carried out using a Valver balloon (18×40 mm). TAVI was performed with the Onecrea valve (26 mm) released under rapid pacing at a depth of 4-6 mm in the LVOT.
Results
The study evaluated the performance of the valve system for the first time in humans. The demographic data are presented in Table 4. All three patients were considered high risk for open surgery, tolerated the TAVI procedure well, and have completed clinical follow-up of 6 months post-procedure at present.
Table 4.
Technical success (at exit from procedure room)
The access, delivery of the device, and retrieval of the delivery system were successfully performed in all three patients. Optimal anatomical positioning of the TAVI was achieved for all the patients and was confirmed by fluoroscopy. There were no device-related complications, and no vascular or structural cardiac complications occurred in the patients studied.
Device success (in hospital)
In addition to freedom from the above-mentioned complications, the intended performance of the valve (mean gradient <13 mmHg, Vmax <2 m/s, and no or trivial PVL) was noted in all three patients. One patient developed a complete AVB noted after the TAVI which necessitated implantation of a permanent pacemaker after 48 hours, after which the patient progressed well and was discharged 24 hours later.
Early safety (at 30 days)
There was no stroke or bleeding (VARC type 2-4); no major vascular, access-related or cardiac structural complication; no acute kidney injury; no moderate or severe AV regurgitation, nor any surgery or intervention directly related to the device.
Clinical efficacy (at 6 months)
The TTE data pretreatment, post-TAVI, at 1 month and 6 months of all three patients are presented in Table 5. Favourable trends were noted in Vmax, MG, PG, and PVL (Figure 5). The usability and ease of operation of the delivery system were found to be acceptable in all three cases.
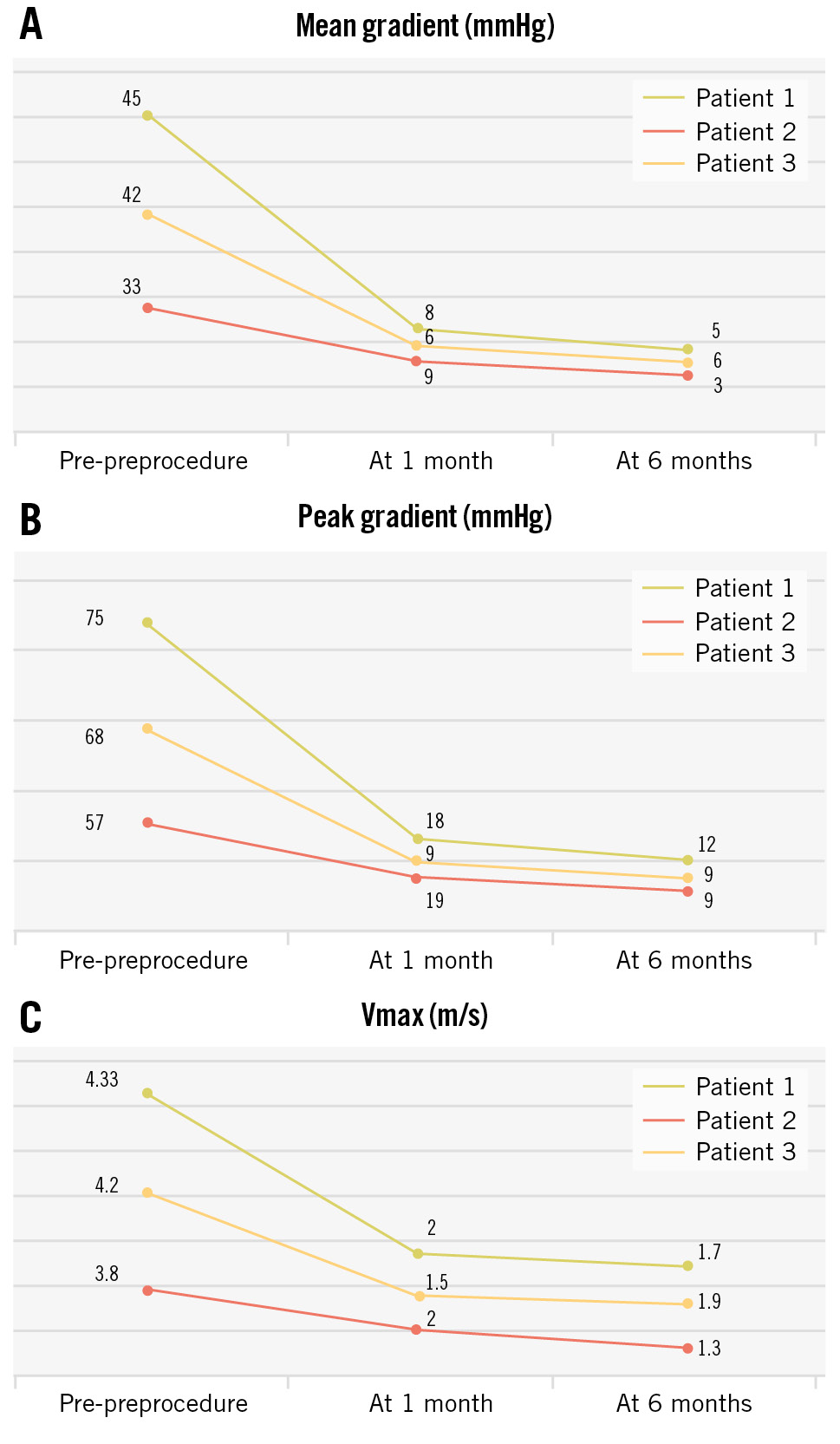
Figure 5. Graphical representation of data from the three patients. The graphs show the reductions in mean pressure gradient (A), peak pressure gradient (B), and Vmax (C). Vmax: maximum velocity
Discussion
This study demonstrates the initial experience with the self-expanding Crea Aortic Valve System in high-risk patients with severe symptomatic AS. The notable findings in this initial experience with the CAV are:
1. Favourable flow haemodynamics after deployment – reduced MG, and Vmax.
2. No PVL or trivial PVL noted on the postprocedural aortogram and follow-up TTE.
3. Reduced peak-to-peak gradient across the aortic valve.
4. Successful implantation in the optimal position in a single attempt.
5. Increased aortic valve EOA post-implantation.
The MG across the aortic valve, which is the integrated gradient throughout the entire systolic ejection period, is the optimal indicator of severity of obstruction7. The MG reduced dramatically after the TAVI procedure in all three patients as seen on the TTE performed during the procedure, after the procedure but before discharge, at 3-4 weeks, and at 6 months. The incidence of paravalvular leak was reported to be 7-40% in previous studies8. Increased severity and the asymmetrical distribution of calcification of the aortic annulus, elliptical and large annular area, and undersizing of the prosthesis are some of the factors contributing to developing a PVL post-TAVI. All three patients studied had severe calcification of the aortic annulus, and the size guide seemed appropriate, thus resulting in no or trivial PVL.
Studies comparing self-expanding (SEV) and balloon-expandable valves (BEV) have shown varying results9. In general, SEV, owing to their supra-annular positioning, allow for a larger EOA. They potentially have a lower incidence of patient-prosthesis mismatch as compared with BEV, which have an intra-annular position. They have a better haemodynamic profile and have shown improvement in left ventricular hypertrophy at 1-year follow-up10. The 5-year outcomes of the CHOICE trial showed comparable clinical outcomes with the two types of valves but better forward haemodynamic flow and a lower incidence of structural valve deterioration in the SEV11. The newer SEV may have better sealing in large annuli and potential advantages in patients with smaller annuli12. The Crea Aortic Valve is designed as a SEV, and the haemodynamic profile and sealing were found to be good in all three patients. The aortic valve EOA, measured using standard transthoracic two-dimensional echocardiography and the continuity equation (EOA=[areaLVOT x VTILVOT ]/ VTIAV), in which VTI is the velocity time integral, was also found to increase during follow-ups in the studied patients, resulting in a good haemodynamic profile comparable to other prosthetic valves in clinical use13. The average EOA increased from 0.63 cm2 before implantation to 1.9 cm2 at 6 months (normal EOA being 3-4 cm2).
In the experience of this study’s team of clinicians, the Crea Aortic Valve System has good usability and seamless delivery, with results comparable to other SEV available at present. All three patients had challenging vascular anatomies for access, with extensive calcification and a large abdominal aortic aneurysm in the first patient and severe calcification and tortuosity in the other two cases. The first patient also had a porcelain aorta, thus posing challenges to vascular access and tracking of the device, and carried an increased risk of stroke1415.
Although all aspects of safety were addressed in this study, further safety data will follow at 1-year post-valve implantation.
Limitations
This study describes the usability, safety, and efficacy of the CAV device in the first three human patients in which it was implanted. However, the completion of phase 1 studies will require a larger sample size. The authors found the performance of the device comparable to other currently available TAVI systems and, therefore, suitable for proceeding with further recruitment of patients. Further studies will be necessary to evaluate the safety and efficacy in high-risk and moderate/low-risk patients as the indications expand.
Conclusions
This is a first-in-human study of Onecrea Medical’s TAVI system after successful preclinical testing. The self-expanding valve has the characteristics required to achieve successful TAVI. Optimal anatomical positioning, expansion of AV area, good reductions in the peak velocity, and MG and PG across the aortic valve with trivial or no paravalvular leak were achieved in a single intervention. There were no incidences of major vascular bleeding nor need for re-exploration or surgical intervention. At 6 months of follow-up, the patients had favourable echocardiographic characteristics and showed good clinical improvement. Serial echocardiography during follow-ups showed further improvements in the expansion of AV area, peak velocity, and mean and peak pressure gradients. Further long-term follow-up studies with larger numbers of patients are needed to establish the safety and performance of Onecrea Medical’s Crea Aortic Valve.
Although further studies are needed to establish the safety and performance of the CAV, the outcome of initial studies is promising, with respect to the CAV being a valve that increases product availability and affordability.
Impact on daily practice
The Crea Aortic Valve is a new transcatheter aortic valve implantation (TAVI) device that has performed well in this study in high-risk elderly patients with severe aortic stenosis. As more and more patients are undergoing TAVI, with a growing geriatric population, newer devices introduced onto the market need close evaluation for safety and efficacy. Optimal anatomical positioning, expansion of aortic valve area, good reductions in the peak velocity, and mean and peak pressure gradients across the aortic valve with trivial or no paravalvular leak in a single intervention are some of the notable features of this system. At 6 months of follow-up, the patients had improved echocardiographic characteristics and showed good clinical improvement.
Funding
Onecrea Medical, Inc. sponsored the study, which was conducted at Policlínica Metropolitana, and its publication.
Conflict of interest statement
All the authors received support and materials for the conduct of the study and preparation of the present manuscript from Onecrea Medical. C. Calderas receives consulting fees and support for travel for relevant meetings from Onecrea Medical. The University of Verona receives financial support from Onecrea Medical through F. Ribichini’s consulting fees and honoraria for lectures and presentations at educational events. M. Taramasso receives consulting fees and support for travel for relevant meetings from Onecrea Medical. The other authors have no conflicts of interest.

