Surgical and transcatheter aortic valve replacement (SAVR and TAVR, respectively) are accepted treatment options for patients with severe aortic stenosis (AS)1. In addition to a significant improvement in survival, both techniques result in a durable improvement in aortic valve haemodynamics following valve replacement234. Whereas data on echocardiographic changes post-SAVR and -TAVR are predominantly based on landmark TAVR clinical trials, corresponding real-world data are limited5. Since patient selection and post-procedural follow-up for patients referred for TAVR in real-world practice may be distinctly different from those in clinical trials, examining changes in left ventricular remodelling and aortic valve haemodynamics post-TAVR and -SAVR in a real-world population will enrich our understanding of the impact of both modalities on functional and geometric measurements and will extend what has already been learnt from randomised clinical trials.
Accordingly, we identified consecutive patients with severe AS referred for TAVR or SAVR at a single centre (Cleveland Clinic Abu Dhabi). Transthoracic echocardiography was routinely performed prior to each procedure (baseline) and at the 6- and 12-month follow-ups. Parameters of aortic stenosis severity, including aortic valve area (AVA) and aortic valve mean gradient, and markers of left ventricular function and size, including left ventricular ejection fraction (LVEF), left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD) and septal wall thickness, were measured at each of the specified time points. Trends in parameters over time, both within and across modalities, were examined using linear mixed models, while accompanying general linear hypothesis tests were used to compare parameter point estimates. Group differences in the magnitude of change across modalities (difference-in-difference [DiD]) in each parameter were calculated as the difference between TAVR and SAVR in parameter change from baseline to 12 months.
Between August 2015 and February 2021, 213 patients underwent valve replacement (137 with TAVR and 76 with SAVR) with a median follow-up of 13 months (interquartile range [IQR] 4-31). Patients referred for TAVR were older (75.6±8.3 years vs 61.6±11.6 years; p<0.001) and had a higher risk of perioperative mortality (STS score 5.8±4.2 vs 2.4±4; p<0.001) and a greater burden of coronary artery disease, diabetes and atrial fibrillation. Furthermore, AS was more advanced at baseline in patients who underwent TAVR, with a smaller AVA (0.7 vs 0.9 cm2; p=0.04) and a lower dimensionless index (0.2 vs 0.3; p=0.01). Otherwise, there were no significant differences in left ventricular or aortic valve measurements between the TAVR and SAVR groups at baseline (Table 1).
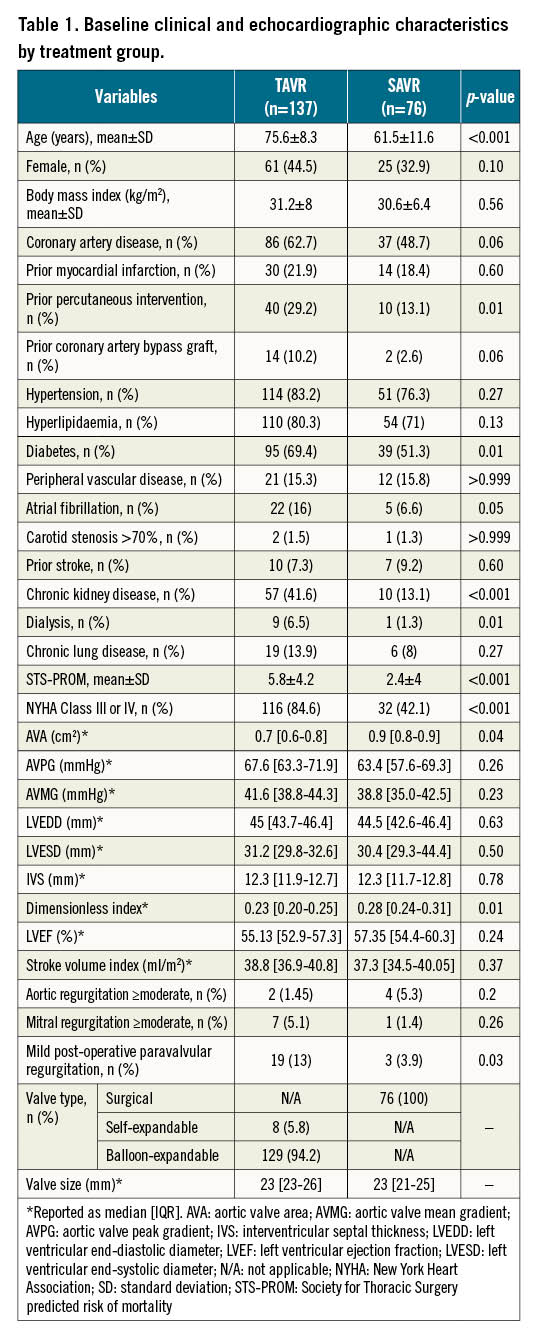
Both groups experienced significant improvement in AVA following valve replacement, with a mean 12-month increase in AVA of 0.5 cm2 (95% confidence interval [CI]: 0.2 to 0.7; p<0.001) and 0.4 cm2 (95% CI: 0.3 to 0.5; p<0.001) in the SAVR and TAVR groups, respectively, and no significant between-group difference (DiD −0.1 cm2, 95% CI: −0.3 to 0.2; p=0.61). Similarly, the aortic valve mean gradient was reduced by 22 mmHg (95% CI: 15.9 to 28.1; p<0.001) and 23.3 mmHg (95% CI: 19.3 to 27.3; p<0.001) in patients who underwent SAVR and TAVR, respectively, with a similar reduction in both groups (DiD 1.3 mmHg, 95% CI: −6 to 8.6; p=0.73). On the other hand, no significant changes were observed in LVEF post-SAVR or -TAVR: a mean change of 2 (95% CI: −1.3 to 5.3; p=0.23) and 0.8 (95% CI: −1.4 to 3; p=0.47) ejection fraction points, respectively, without a statistically significant difference across the groups (DiD −1.2%, 95% CI: −5.2 to 2.7; p=0.55).
Furthermore, both groups experienced a similar reduction in septal wall thickness (mean reduction of 1.1 mm [95% CI: 0.3 to 1.8] and 0.7 mm [95% CI: 0.2 to 1.2] in SAVR and TAVR, respectively; p=0.01 for both comparisons; DiD −0.4 mm [95% CI: −1.3 to 0.6; p=0.47]). SAVR, but not TAVR, was associated with a more pronounced reduction in LVEDD (mean reduction of 2.8 mm [95% CI: 0.8 to 4.8] and 0.5 mm [95% CI: −0.8 to 1.9]; p=0.01 and 0.44, respectively). However, this difference did not reach nominal statistical significance (DiD −2.3, 95% CI: −4.6 to 0.1; p=0.07). Similarly, LVESD changed significantly after SAVR (mean reduction 2.2, 95% CI: 0.3 to 4.1; p=0.02), while LVESD did not change significantly post-TAVR (mean reduction 0.7 mm, 95% CI: −0.6 to 2.0; p=0.27), with no significant between-modality difference (p=0.2) (Figure 1).
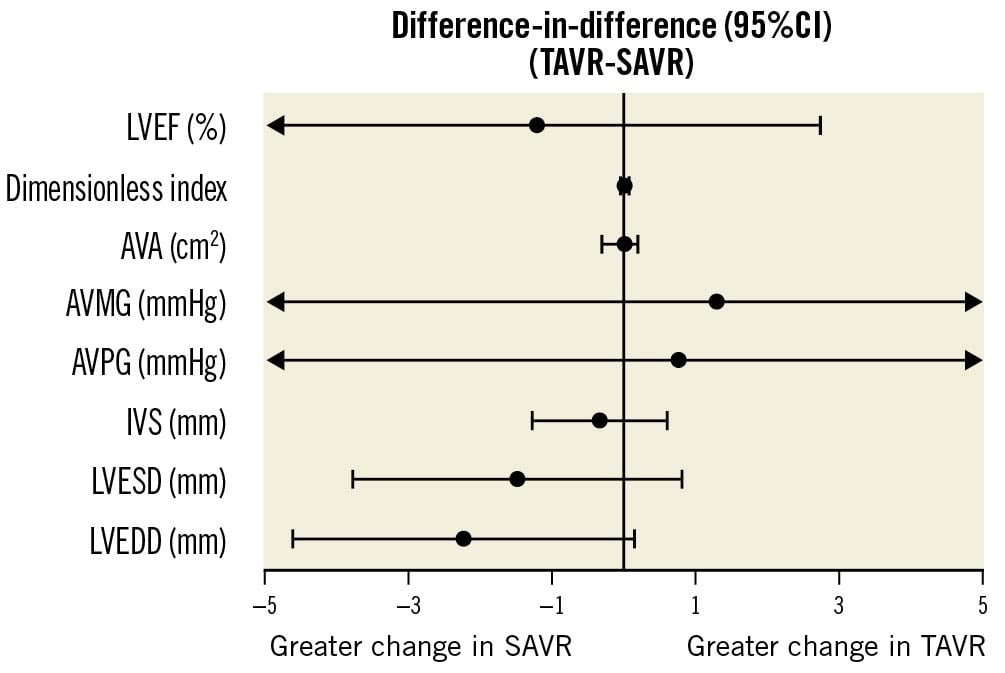
Figure 1. Comparative changes in haemodynamics and ventricular measurements in TAVR vs SAVR. This figure represents the differences in the magnitude of change across modalities (difference-in-difference [DiD], TAVR-SAVR ) in each parameter, between baseline and 12-month follow-up. AVA: aortic valve area; AVMG: aortic valve mean gradient; AVPG: aortic valve peak gradient; IVS: interventricular septal thickness; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; SAVR: surgical aortic valve replacement; TAVR: transcatheter aortic valve replacement
The current findings mirror those from echocardiographic analyses of randomised studies, showing significant and comparable improvement in aortic valve haemodynamics and evidence of reverse left ventricular remodelling with TAVR and SAVR234, and add to the available, albeit limited, real-world data on long-term echocardiographic findings with the 2 modalities5. Despite the single-centre nature and the small sample size in this study, the current findings are in line with previous literature and confirm a favourable performance of both TAVR and SAVR in improving echocardiographic parameters of AS and in reversing markers of adverse left ventricular remodelling in unselected patients referred for valve replacement.
The observation that left ventricular dimensions had changed more favourably post-SAVR may appear discrepant with current literature. However, this may potentially be due to preferential treatment of patients with more severe AS, and hence more advanced ventricular remodelling at baseline, with TAVR in this non-randomised study. As a result, these patients were less likely to experience improvement in left ventricular geometry compared to those treated with SAVR, who had less advanced remodelling at baseline. Comparing changes in mitral and aortic regurgitation following valve replacement would further enrich this analysis; however, this was unfortunately not feasible due to the limited availability of necessary data.
In conclusion, both TAVR and SAVR result in comparable improvement in both the echocardiographic parameters of AS and the markers of adverse left ventricular remodelling in unselected patients referred for valve replacement.
Conflict of interest statement
A. Edris serves as a proctor for Edwards Lifesciences. The other authors have no conflicts of interest to declare.

