Introduction
The first drug-eluting stents (DES) were introduced in 2003 as an alternative to bare metal stents (BMS) and have demonstrated reduced risk of restenosis in percutaneous coronary interventions (PCI) compared to BMS, balloon angioplasty and vascular brachytherapy1,2. Design iterations have produced “second-generation” DES to improve deliverability and biocompatibility and thus lessen the inflammatory response after intervention. Several studies have reported an improved safety profile with second-generation DES compared to first-generation DES and BMS3,4. The second-generation Resolute™ and Resolute Integrity™ DES (Medtronic, Minneapolis, MN, USA) have been studied in multiple prospective clinical studies with over 7,600 patients enrolled worldwide in the RESOLUTE Global Clinical Program. Long-term follow-up data on DES patients are important to illustrate the continued safety and efficacy of the intervention. Five-year clinical outcomes have been published from several studies in the RESOLUTE Global Clinical Program5,6, but more long-term outcome data are needed specifically for the Asian population. The current report presents 5-year clinical outcomes for the RESOLUTE China Registry (R-China Registry).
Methods
STUDY DESIGN
The study design of the R-China Registry has been reported previously7,8. Briefly, the R-China Registry is a prospective, multicentre, observational study in an all-comers Chinese population. Patients were 18 years or older and acceptable candidates for PCI, with minimal exclusion criteria. After implantation with Resolute DES, patients were followed at 30 days, 6 months, and annually to 5 years by office visit or phone call. At each follow-up, data on adverse events and dual antiplatelet therapy (DAPT) usage were collected. The trial complies with the Declaration of Helsinki, local ethics committees approved the clinical protocol at each enrolling centre, and informed consent was obtained from all patients.
Study oversight was provided by the steering committee comprised of physician investigators and sponsor representatives. Safety oversight was provided by an independent data safety monitoring board. Outcomes were adjudicated by a clinical events committee of cardiologists not participating in the clinical study. Monitoring by R&G Pharma Studies (Shanghai, China) was conducted at all study centres to verify source data from at least half of all enrolled patients, and the source was verified for all serious adverse events.
STATISTICAL ANALYSIS
The primary endpoint was one-year target lesion failure (TLF), defined as a composite of cardiac death, target vessel myocardial infarction (MI; Q-wave and non-Q-wave) or clinically driven target lesion revascularisation (TLR). The main secondary endpoint was overall stent thrombosis at one year, defined as definite or probable stent thrombosis, according to the Academic Research Consortium (ARC) definition. The following secondary endpoints were assessed annually up to 5 years: TLF, all deaths, stent thrombosis rates, MI, TLR, clinically driven target vessel revascularisation (TVR), significant bleeding complication, and target vessel failure (TVF). TVF is a composite endpoint of cardiac death, target vessel MI (TVMI), or TVR. Significant bleeding complication is defined as bleeding that led to an interruption of antiplatelet medication, required transfusion, resulted in substantial haemodynamic compromise requiring treatment, or intracerebral bleeding. Deaths were considered cardiac unless an unequivocal non-cardiac cause could be established. All MI, including TVMI, were adjudicated according to the extended historical definition9.
Clinical outcomes at 5 years were assessed for the following pre-specified subgroups: treatment of long lesions (≥18 mm length), small vessels (≤2.75 mm diameter), multiple vessels, chronic total occlusions (CTO), overlapping stents and bifurcations, as well as treatment in patients with acute myocardial infarction (AMI) within 72 hours or a history of diabetes mellitus. Clinical outcomes at 5 years in complex patients were also analysed, where complex patients were defined as having a bifurcation, bypass grafts, in-stent restenosis, AMI within 72 hours, left ventricular ejection fraction <30%, unprotected left main coronary artery, more than two vessels stented, renal insufficiency or failure (creatinine >140 µmol/L), lesion length >27 mm, more than one lesion per vessel, lesion with thrombus or total occlusion (pre-procedure Thrombolysis In Myocardial Infarction [TIMI]=0).
All analyses were conducted based on the intention to treat; no data imputation for missing values was performed. Nominal variables are presented as percentages and continuous variables as mean±standard deviation. The counts and percentages of clinical events are presented in table format and the incidence of clinical events is presented using the Kaplan-Meier method in figure format. Statistical analyses were performed using SAS software, version 9.1 or later (SAS Institute, Cary, NC, USA).
Results
From December 2010 to March 2012, 1,800 patients were enrolled in the R-China Registry at 30 clinical study centres in China. Baseline and procedural characteristics have been reported previously and are shown in Table 1 and Table 2,7,8. Percentages of patients with various comorbidities or lesion/procedural complexity are included in Table 3. There were 61% complex patients, 29% of patients with a history of diabetes, 68% with long lesions and 43% with small vessels. DAPT usage for the R-China Registry was 39%, 36% and 35% at 3, 4, and 5 years post procedure, respectively (Figure 1). The rate of reported significant bleeding events at 5 years was 2.2%. The follow-up visit compliance rate for 5-year follow-up was 88.1%.
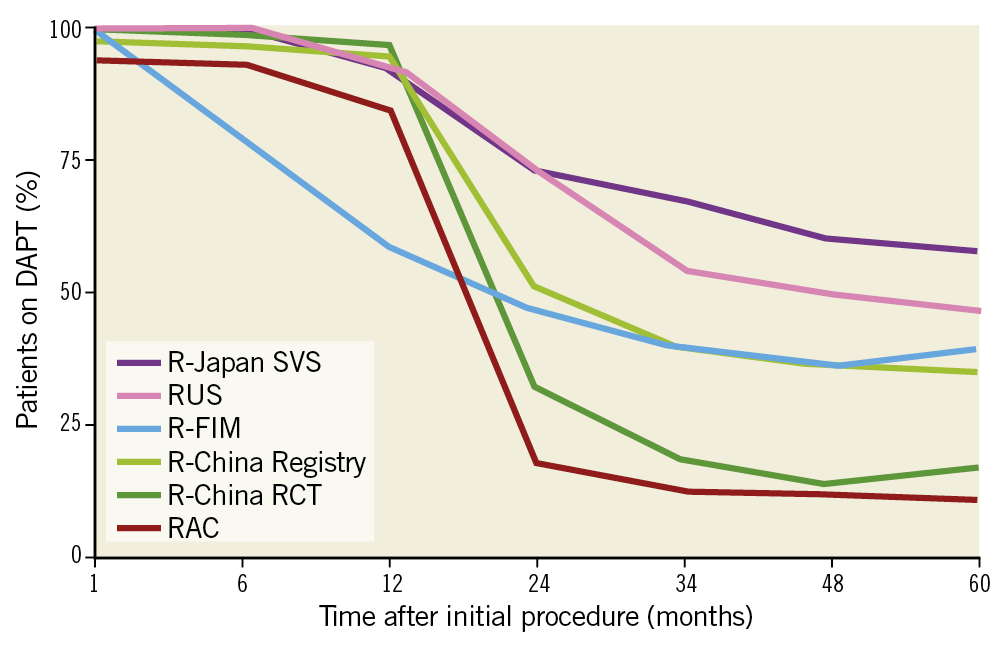
Figure 1. DAPT usage at 5 years in the RESOLUTE Global Clinical Program. DAPT: dual antiplatelet therapy; FIM: first in man; RAC: RESOLUTE-III All-comers Trial; RCT: randomised clinical trial; SVS: small vessel substudy
Clinical outcomes and rates of stent thrombosis at 5 years are shown in Table 4. At 5 years, TLF was 9.8%, cardiac death 3.5%, TVMI 3.2% and TLR 4.6%. The rate of very late stent thrombosis at 5 years was 0.5%. The cumulative incidence of events using Kaplan-Meier analysis is shown for TLF, TLR and stent thrombosis for 0 to 5 years in Figure 2. Rates of TLF, cardiac death/TVMI, TLR and stent thrombosis at 5 years were analysed for pre-specified subgroups (Figure 3). The rates of TLF across multiple all-comer clinical studies in the RESOLUTE Global Clinical Program are compared from 0 to 5 years (Figure 4).
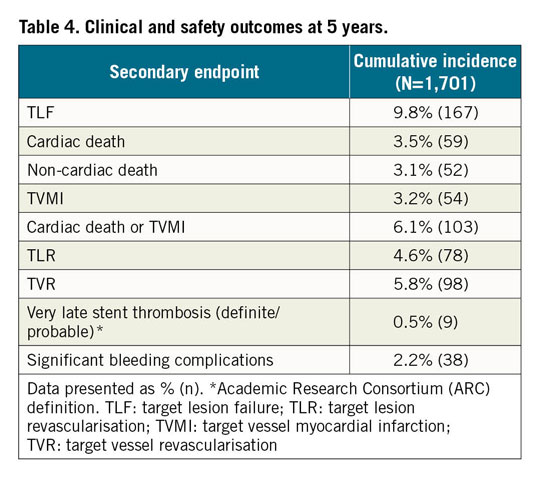
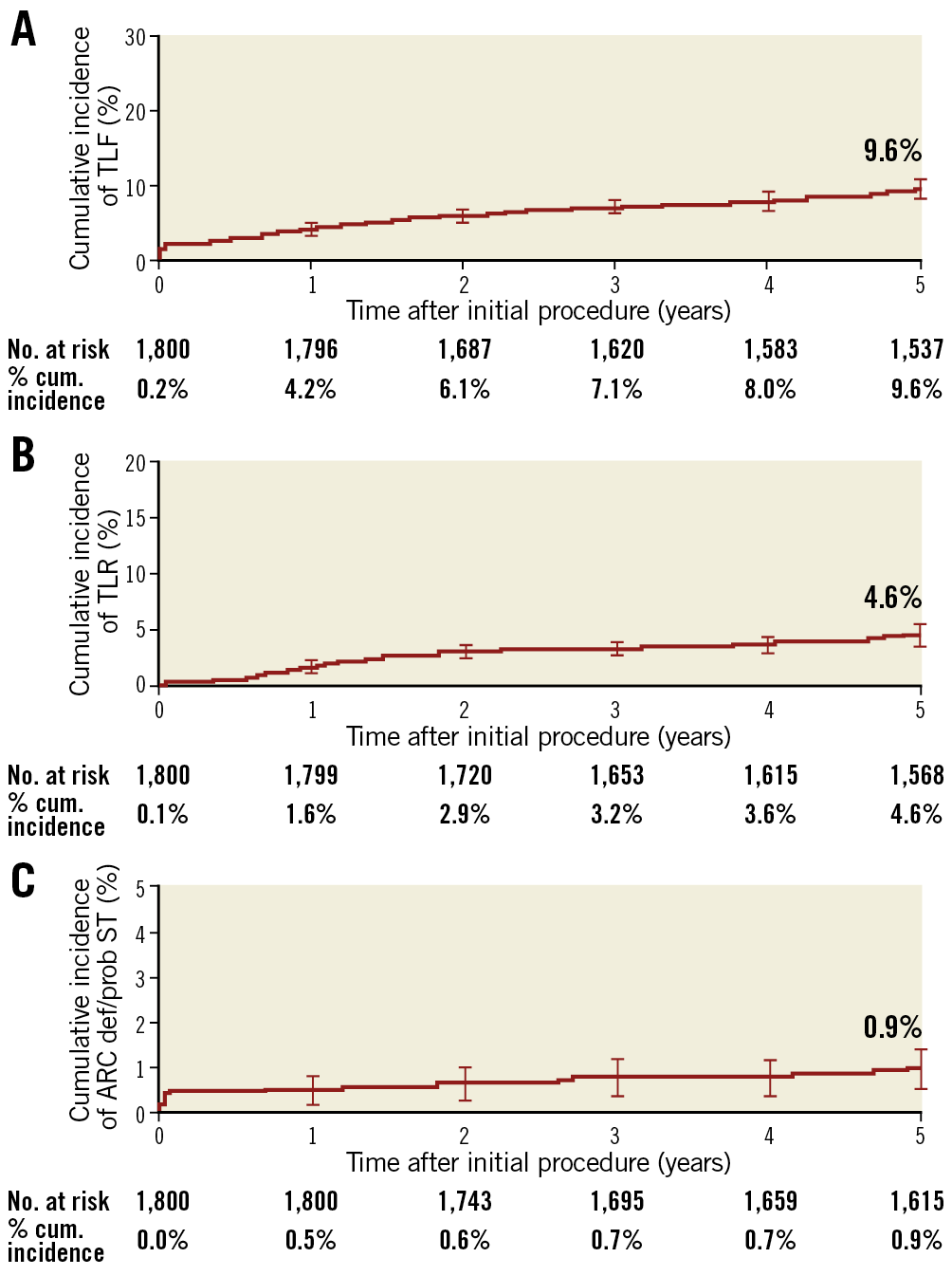
Figure 2. Cumulative incidence of events using Kaplan-Meier analysis to 5 years. A) TLF. B) TLR. C) Stent thrombosis. cum.: cumulative; ST: stent thrombosis; TLF: target lesion failure; TLR: target lesion revascularisation
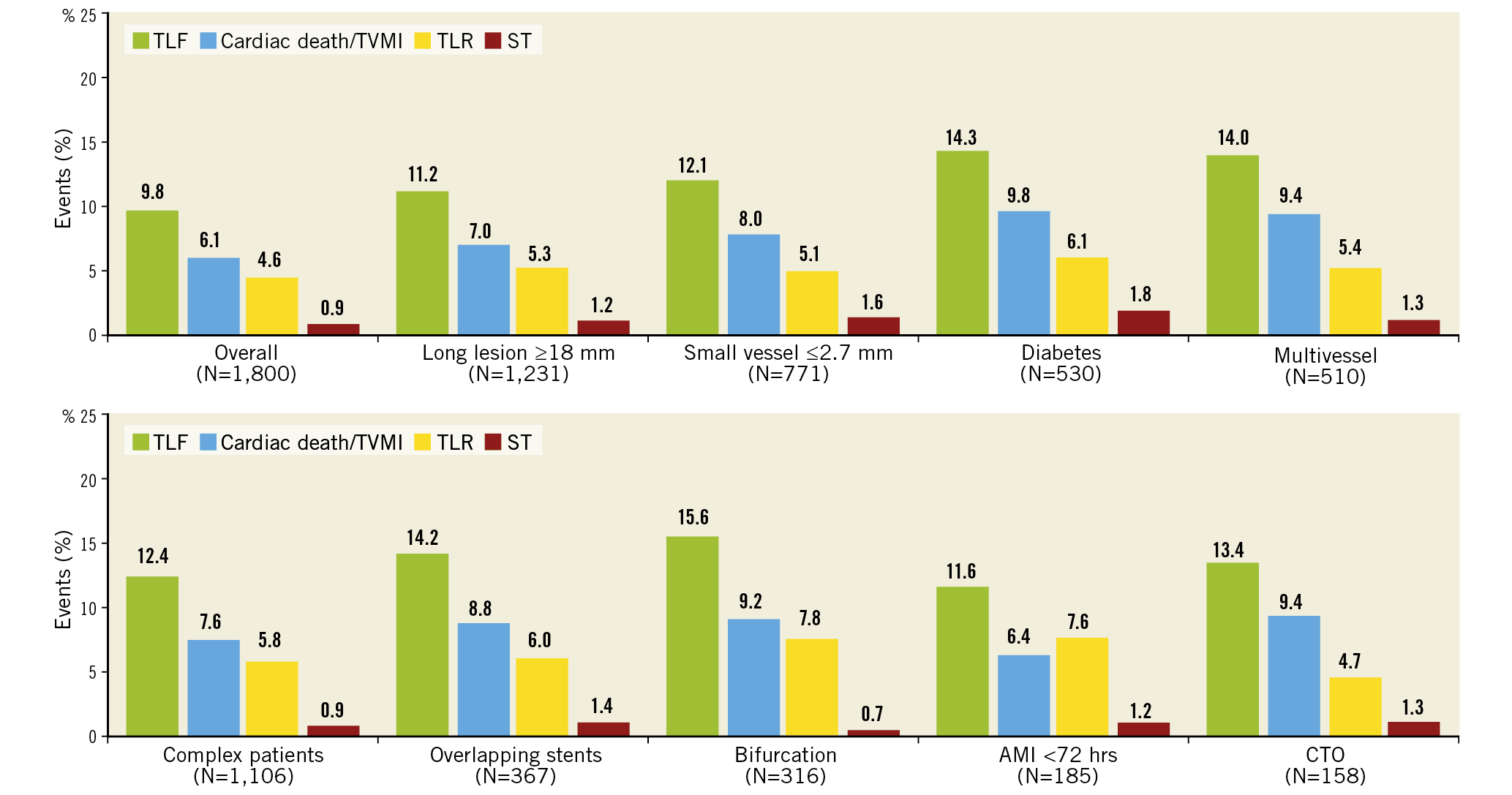
Figure 3. Subgroup analyses: cumulative incidence of secondary endpoints at 5 years. AMI: acute myocardial infarction; CTO: chronic total occlusion; ST: stent thrombosis; TLF: target lesion failure; TLR: target lesion revascularisation; TVMI: target vessel myocardial infarction
Figure 4. Cumulative incidence up to 5 years after Resolute DES implantation of TLF in RESOLUTE-III All-comers, RESOLUTE China Randomized Controlled Trial and RESOLUTE China Registry.
Discussion
Results at 5 years in the R-China Registry include low rates of TLF and other secondary endpoints in a trial where 61% of patients were complex, illustrating the robust safety and efficacy profile of Resolute DES in this all-comers Chinese population.
Other DES trials to report 5-year clinical outcomes include COMPARE II10, SPIRIT III11 and TWENTE12. The rates of 5-year TLF were 13.4% (biolimus-eluting stents [BES]) vs 11.5% (everolimus-eluting stents [EES]) for COMPARE II, 12.7% (EES) vs 19.0% (paclitaxel-eluting stents [PES]) for SPIRIT III and 15.0% (Resolute DES) vs 16.2% (EES) for TWENTE10,11,12. These 5-year TLF rates are all higher than the TLF rate in the R-China Registry (9.8%), driven by a low TLR rate of 4.6%. As the R-China Registry was an all-comers registry, patient selection bias and lack of mandated angiographic follow-up could have influenced the TLR rate; however, long-term outcome data after PCI in the Chinese population are limited, so this is speculative.
The TLF rate for the R-China Registry compared to other Resolute clinical studies with 5-year follow-up was similar to the TLF rate of 10.3% for Resolute patients in the R-China randomised controlled trial (RCT) but less than the rate of 17.1% for Resolute patients in the RESOLUTE-III All-comers (RAC) trial (Figure 4). There was a higher proportion of complex patients in RAC compared to the R-China Registry (67% vs 61%, respectively), which could have contributed to the difference in TLF rates. The TLF rate reported for pooled Resolute trial data was midway between these rates at 13.4%6. The TLF rate for RESOLUTE Japan, included in the pooled analysis of Resolute studies, was also low with a rate of 5.6% at 4 years13. Reports of differences in thrombotic and thrombolytic status for Asian patients versus patients in Western countries have been postulated as reasons for differences in the underlying causes of thrombotic events14,15.
Patients with small vessels (reference vessel diameter [RVD] <2.75 mm) experienced a TLF rate of 12.1% and a TLR rate of 5.1% at 5 years, higher than for the overall cohort in the R-China Registry. Limited data exist on long-term follow-up of patients with small vessel disease after DES implantation. The PERSEUS Small Vessel trial reported a high TLF rate of 20.1% and a TLR rate of 13.6% at 5 years for lesions of RVD between 2.25 and 2.75 mm16. Historically, PCI in small vessels has resulted in an increase in adverse events17. However, several recent studies have reported no differences in clinical outcomes or angiographic measurements for small (RVD ≤2.5 mm) compared to non-small vessels after DES implantation, including no association of increased major adverse cardiac events (MACE) or TVR at 1 year18, no difference in early or late restenosis at 8 months19, and no difference in TLR at 2 years20 for treatment in small vessels. Notably, favourable short-term outcomes were reported for a subsequent iteration of the R-ZES, the Resolute Onyx™ ZES (Medtronic), for patients with very small (RVD <2.25 mm) lesions: 5.0% TLF and 2.0% TLR at 1 year21, suggesting that advances in stent technology may ameliorate the performance of DES in small vessels after PCI.
Adverse event rates were higher for R-China Registry patients in pre-specified subgroups compared to the overall cohort. Specifically, TLF was 14.3% and TLR 6.1% in diabetic patients at 5 years (Figure 3). To our knowledge, this is the only 5-year clinical study follow-up reported for Chinese diabetic patients post DES implantation, although short-term outcomes have been published. A large single-centre non-randomised Chinese study comparing outcomes in diabetic versus non-diabetic patients found that diabetic patients had worse outcomes including death, MI, revascularisation, and MACE at 2 years22. However, despite the increase in adverse events, diabetic status was not an independent predictor of mortality after propensity score matching22. The multicentre RESOLUTE-CHINA DIABETES study demonstrated favourable outcomes in diabetic patients implanted with Resolute DES23.
DAPT usage has varied widely across Resolute clinical studies (Figure 1), ranging from 11% to 62.5% at 5 years. DAPT prescription rates were generally higher in the US and Japanese trials (R-38, R-Japan SVS and RUS), whereas, in the European-based RAC trial, DAPT prescription was lowest despite relatively high lesion and patient complexity. DAPT usage in the R-China Registry was 35% at 5 years and bleeding events were rare (2.2%). While European Society of Cardiology and American College of Cardiology/American Heart Association guidelines recommend at least 6 months DAPT for newer-generation DES in stable patients without high bleeding risk, there is limited consensus for the optimal duration of DAPT in patients with high bleeding risk after PCI24,25. Recent and ongoing studies are being conducted to evaluate the potential of shortened (1-month) DAPT in high bleeding risk patients26,27,28. The rate of definite/probable stent thrombosis in the R-China Registry was 0.5% at 5 years and similar to rates in other published studies5,6,10,11,12.
Limitations
As in other registries, there was no control group in this trial. Intravascular ultrasound and optical coherence tomography data were not collected since these imaging procedures were not in common practice in China when the study was enrolling patients. Although not all patient data were monitored in the R-China Registry, an analysis at one year found no difference in outcomes between monitored and unmonitored patients7. Subgroup analyses were pre-specified, but randomised trials are needed to confirm the findings. Results from this study may be specific to China and may not necessarily be indicative of outcomes in other Asian or Western countries.
Conclusions
The RESOLUTE China Registry is the largest study of Asian patients treated with second-generation Resolute DES. Long-term follow-up to 5 years shows overall low clinical event rates. At 5 years, 35% of patients remained on DAPT. Clinical outcomes illustrate a robust safety and efficacy profile of Resolute DES in a real-word Asian population, including favourable performance in complex patient subsets.
Impact on daily practiceLong-term outcomes after percutaneous coronary intervention are needed to demonstrate the safety and efficacy of drug-eluting stents. The results of the R-China Registry show the continued safety and efficacy of the Resolute drug-eluting stent at 5 years in a Chinese population. |
Acknowledgements
Lisa Bousquette, MS, and Linda Zhi provided expert study management (both employees of Medtronic).
>Funding
The RESOLUTE China Registry (ClinicalTrials.gov NCT01243749) was funded by Medtronic.
Conflict of interest statement
B. Ferri and M. Liu are employees of Medtronic. The other authors have no conflicts of interest to declare.

