Introduction
The clinical entity of atrial functional tricuspid regurgitation (AF-TR) is now increasingly recognised as having distinct echocardiographic features of annular dilatation accompanying right atrial enlargement in the presence of atrial fibrillation1. Severe symptomatic forms of AF-TR are not uncommon and are associated with high perioperative mortality if repaired surgically2. Fortunately, transcatheter options are now available and have recently been incorporated into treatment algorithms as per guidelines23. To date, the two main approaches for percutaneous TR treatment are repair (leaflet approximation or annuloplasty) and replacement (orthotopic or heterotopic)4. Heterotopic caval valve implantation using the TricValve® (OrbusNeich P&F) is a unique interventional approach for treatment of severe AF-TR in patients who are deemed ineligible for surgery. The concept behind this approach is to reduce caval backflow, hence reducing systemic venous congestion promoting right ventricular remodelling and increasing cardiac output. The success of the first-in-man application of this approach5 spurred us to pursue such a treatment for our patient with the aim of improving quality of life. The following case was successfully treated with the TricValve.
Methods
A 67-year-old female patient presented with worsening dyspnoea and reduced effort tolerance (New York Heart Association [NYHA] Functional Class III). Clinically, there was elevated jugular venous pressure (JVP) and peripheral oedema with elevated N-terminal (NT)-proB-type natriuretic peptide (NT-proBNP) of 5,648 pg/ml despite being on optimised medical therapy, including diuretics. Her 6-minute walk test (6MWT) was 320 meters. The patient had underlying atrial fibrillation and was on novel oral anticoagulation. She also had hypertension and dyslipidaemia. The patient’s estimated glomerular filtration rate (eGFR) was 52 ml/min/1.73 m2. Transthoracic echocardiography (TTE) revealed severe tricuspid regurgitation (TR) with normal valve morphology, annular dilatation of 4.6 cm, poor coaptation of valve leaflets (coaptation gap of 4 mm from TTE apical four-chamber [A4CH] view) and hepatic vein systolic flow reversal seen. Only mild functional mitral regurgitation (MR) was present. The basal diameter of the right ventricle (RV) was 4.9 cm. Right ventricular function was still preserved with a tricuspid annular plane systolic excursion (TAPSE) of 1.5 cm (reduced from 2.1 cm, 2 years ago). The left ventricular ejection fraction (LVEF) was 38% (reduced from 60%, 2 years ago) (Moving image 1–Moving image 3). This could be explained by her chronic hypertension as well as biventricular failure due to untreated severe TR. Right heart catheterisation showed a mean pulmonary artery pressure of 49 mmHg with a pulmonary capillary wedge pressure of 18 mmHg and an elevated pulmonary vascular pressure of 3.51 Wood units. The calculated EuroSCORE risk was 2.24%. A computed tomography pulmonary angiogram and high-resolution computed tomography were performed showing normal lung parenchyma and no evidence of pulmonary artery thromboembolism. The coronary angiogram showed mild to moderate coronary artery disease.
The patient was repeatedly counselled for surgery but the patient refused. She was then referred to the Heart Team for consideration and evaluation for percutaneous bicaval valve implantation. This transcatheter treatment method was selected in view of her advanced tricuspid valve disease and because it was the only non-surgical option available in the region. At this time, TriClip (Abbott) was not yet available in the region. The chosen device, TricValve, has two self-expanding biological valves with nitinol frames that are implanted into the superior vena cava (SVC) and inferior vena cava (IVC) without disturbing the native tricuspid valve (Figure 1).
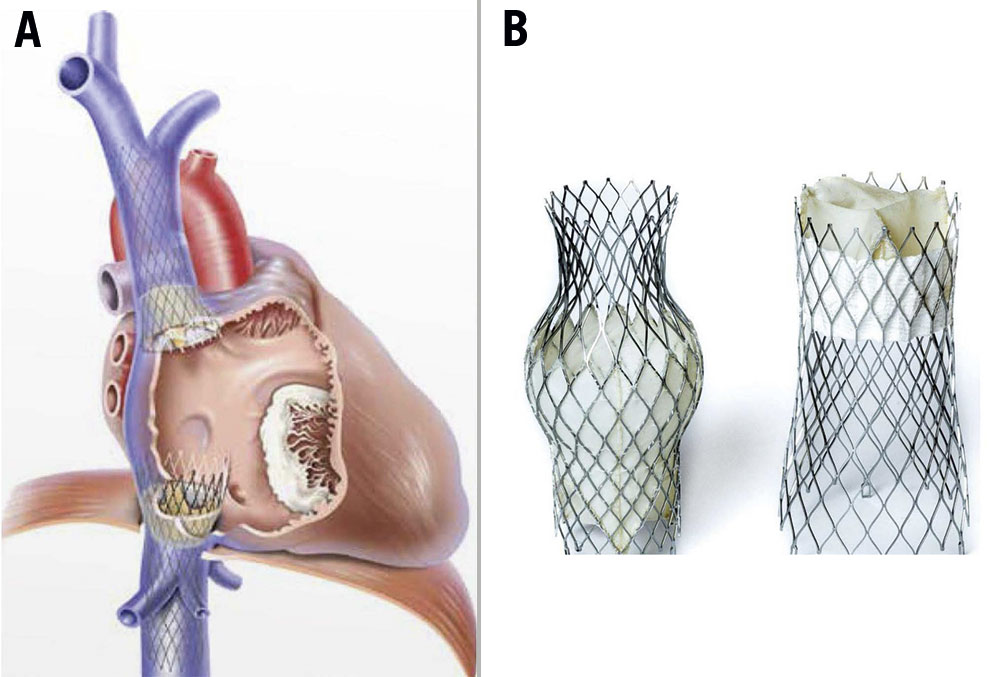
Figure 1. TricValve illustration. A) Post-implantation. B) TricValve model.
Prior to procedure, the patient underwent a computed tomography angiogram (Figure 2). The patient was then deemed suitable for the TricValve with a calculated SVC size of 25 mm and an IVC size of 31 mm. A Heart Team meeting was organised. She was electively intubated and the intervention (Moving image 4) was conducted in the hybrid operating theatre under general anaesthesia with fluoroscopic guidance. As this was the first case performed in our region, the entire procedure was executed under virtual proctorship of experienced operators from Austria and Spain.
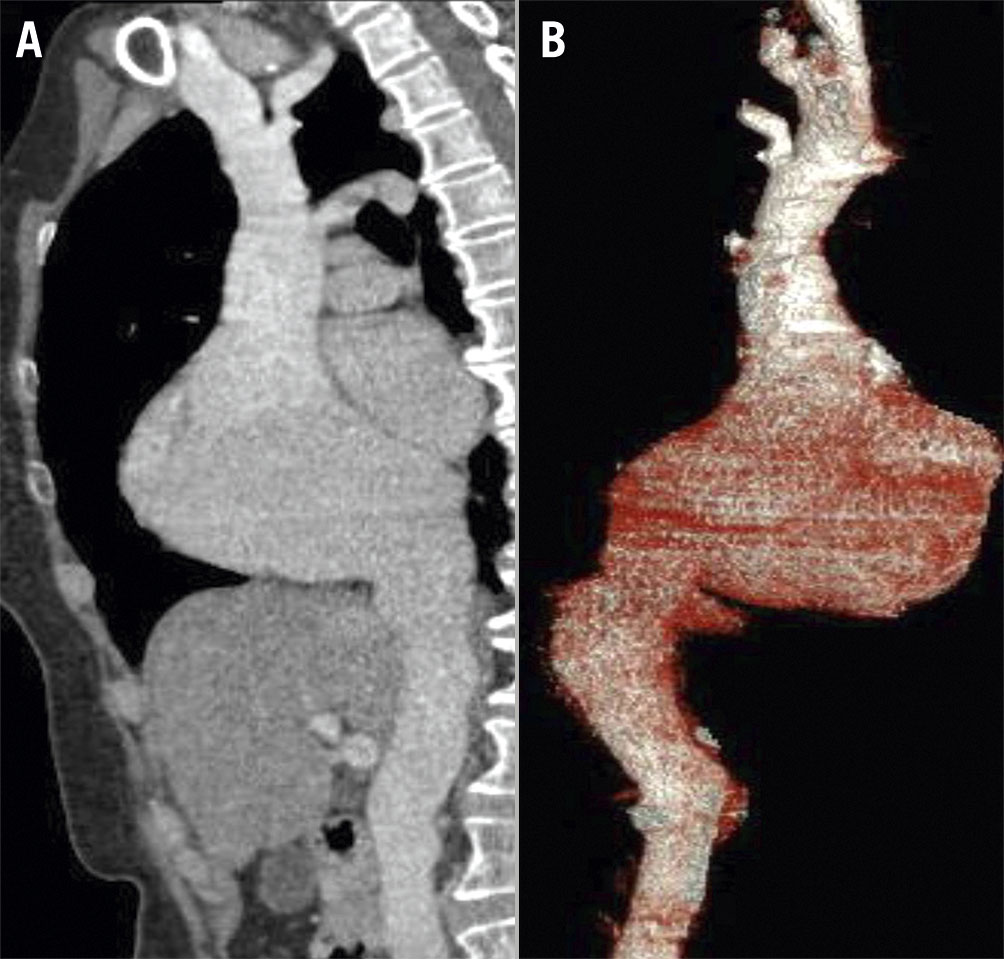
Figure 2. Pre-interventional procedural planning (multimodality imaging). A) Computed tomography angiogram and B) 3D reconstruction showing the superior vena cava and inferior vena cava anatomy to assess suitability for delivery of valves and to allow for measurements/sizing.
After anaesthetic induction, the patient was placed on low doses of noradrenaline and milrinone infusion as inotropic support with nitric oxide. The anaesthesia team was monitoring the patient throughout the procedure. Transoesophageal echocardiography (TOE) was done to reconfirm previous findings. Transfemoral access was established. A 22 Fr sheath was utilised for right venous access. A Lunderquist extra-stiff wire (Cook Medical) was placed at the right internal jugular vein. A 6 Fr pigtail catheter was then advanced to the junction of the innominate and subclavian vein which was used as landmark, and a multipurpose catheter (MPA) was advanced to the right pulmonary artery (rPA) to mark the crossing of the rPA with the SVC via the left femoral vein. The sheath was then exchanged to insert the SVC bioprosthesis into the junction, with the belly of the prosthesis positioned above the rPA crossing. The catheter position was confirmed under fluoroscopy with TOE guidance before being slowly deployed. The TricValve delivery system was retrieved and the MPA catheter was withdrawn. Next, the IVC bioprosthesis was loaded onto the delivery system and positioned to be inserted at the height of the diaphragm with the skirt visible just above the hepatic vein. The position was verified mainly with the aid of TOE using the bicaval view (Figure 3). Final placement was confirmed with the skirt noted to be 20 mm into the right atrium (RA). No paravalvular leaks were noted with either bioprosthesis. The procedure took roughly 2 hours. After implantation, invasive measurements showed a mild reduction of pressures and the patient was weaned off inotropes within the next 24 hours in ICU. The post-placement chest radiograph is shown in Figure 4. The patient was subsequently extubated after 36 hours. Figure 5 shows the before and after placement of the TricValve system.
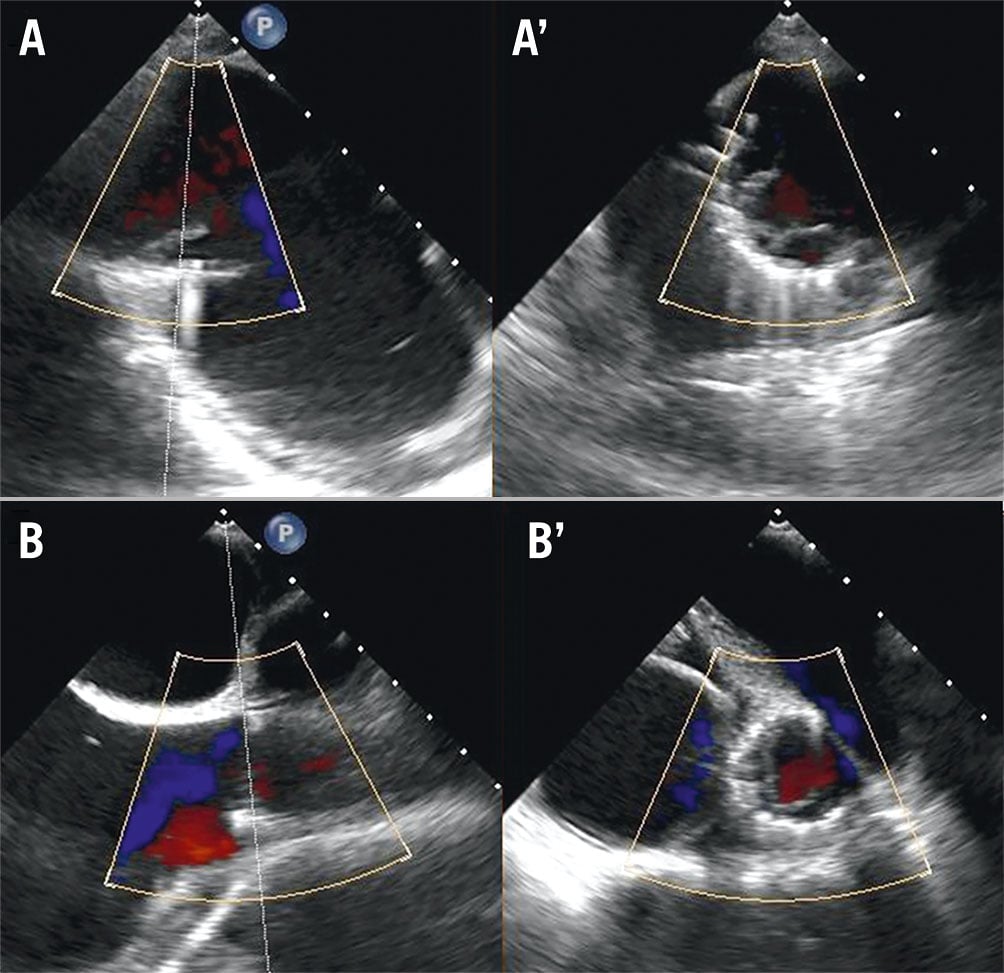
Figure 3. Post-procedural transoesophageal echocardiography using bicaval views with X-plane imaging showing placement of both valves (A, A’: IVC, and B, B’: SVC) with no paravalvular leakage seen. IVC: inferior vena cava; SVC: superior vena cava
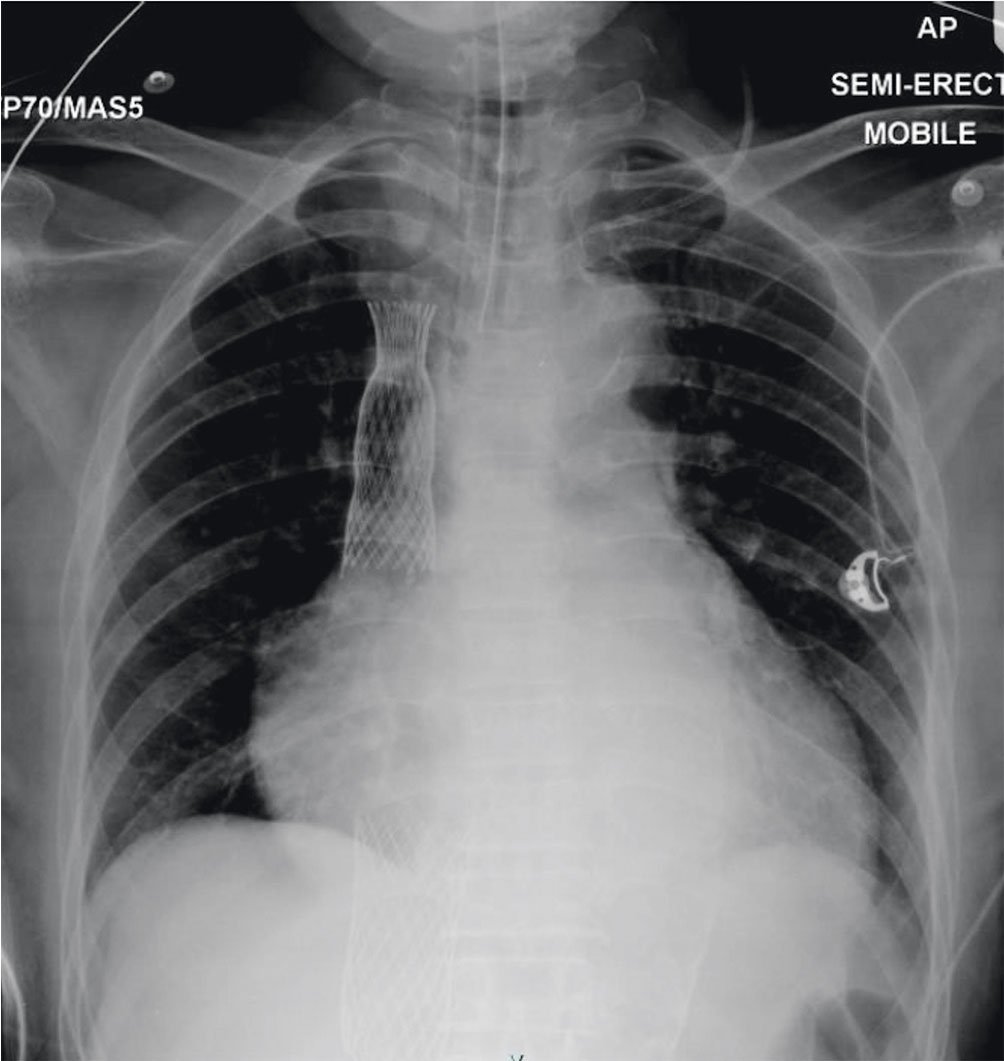
Figure 4. Post-procedural chest radiography showing placement of the SVC and IVC valves. Cardiomegaly is also present with a cardiothoracic ratio of 0.72. IVC: inferior vena cava; SVC: superior vena cava
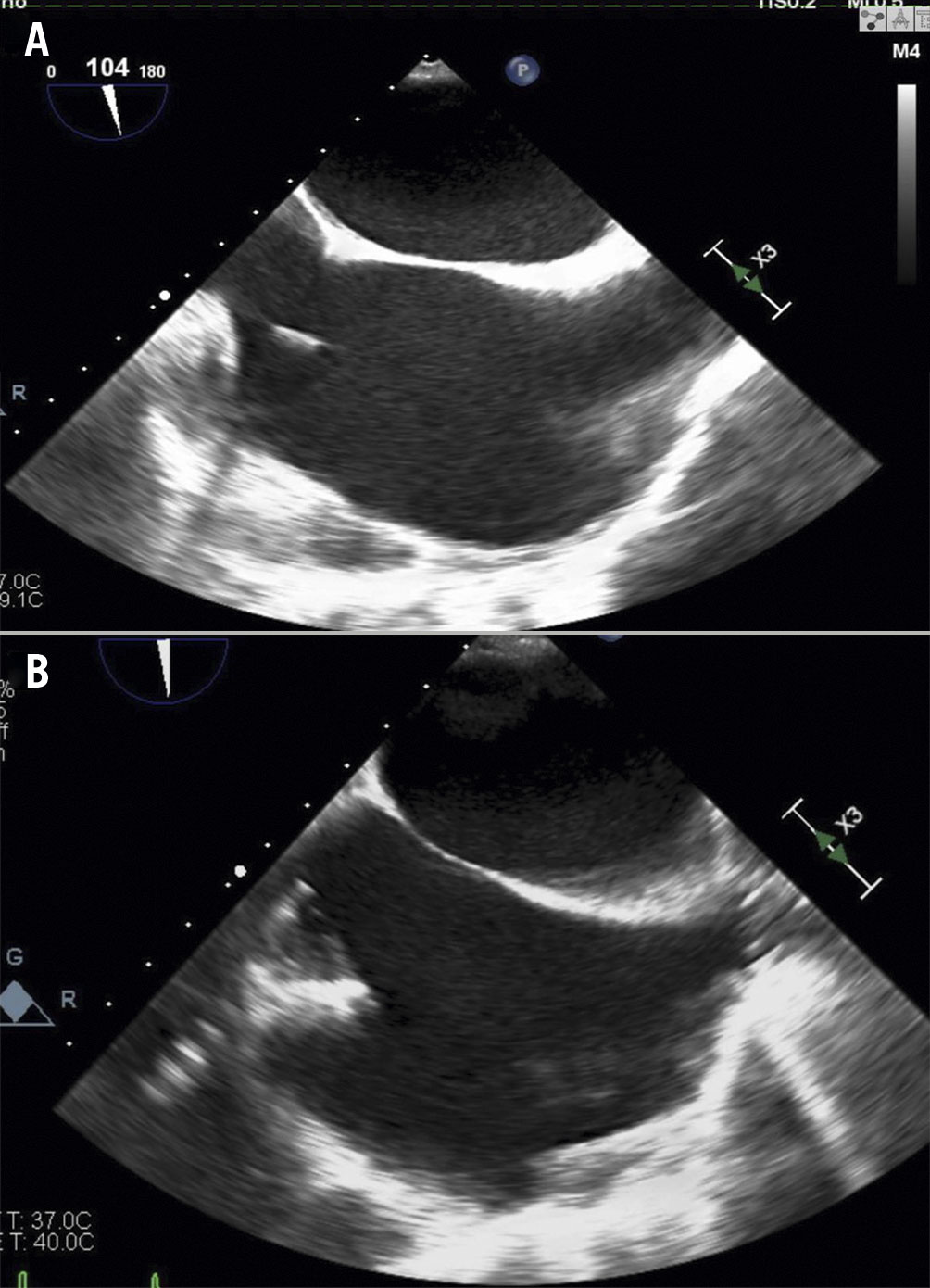
Figure 5. Post-interventional transoesophageal echocardiography using bicaval views showing before (A) and after (B) placement of the TricValve.
Results
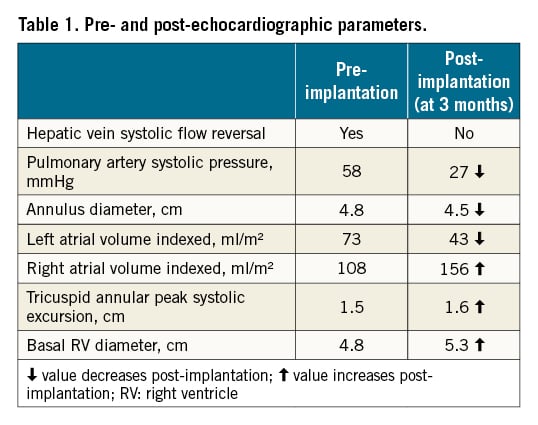
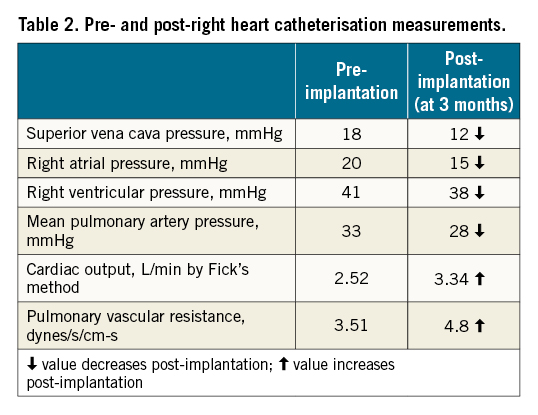
The patient was discharged on the fifth day after the procedure. The patient did not experience any adverse reaction to the procedure. She was followed up at 1 and 3 months. Clinically, she gradually improved (NYHA Functional Class I to II). Her NT-proBNP levels markedly declined to 3,963 pg/ml (at 1 month) and subsequently to 2,072 pg/ml (at 3 months). Her 6MWT also improved to 420 meters. Echocardiographic parameters also improved (Table 1) except for the left ventricular systolic function which remained the same. Invasive haemodynamic monitoring is shown in Table 2. Most parameters did improve except for the RA volume and RV diameter, which increased. This represents the variability that is noticed in patients post-TricValve implantation. However, as the patient is improving clinically, a longer time is likely required for favourable cardiac remodelling to take place. Anticoagulation with a vitamin K antagonist was planned for 1 year as there are still limited data on the efficacy of the use of direct oral anticoagulants (DOAC).
Discussion
To the best of the authors’ knowledge, this is the first successful case of TricValve implantation in Asia for a patient with worsening RV dysfunction who declined surgery. The TricValve stands out from others in that only a small part of the valve protrudes out from the IVC, hence there is minimal foreign material in the right atrium. Also, two separate valves are necessary to diminish the congestion completely, hence promoting positive haemodynamic changes. It is important to have good anaesthesia support too, such as in our case emphasising the use of nitric oxide, pulmonary vasodilators and appropriate inotropes for RV failure6. With regard to the coaptation gap, this echocardiographic parameter is not needed in determining the suitability of a patient for the TricValve system.
The TRICUS STUDY – Safety and Efficacy of the TricValve® Device (Clinicaltrials.gov: NCT03723239) is an ongoing trial to investigate further the safety and efficacy of the TricValve device. As per the trial guidelines, we initially screened for patients with severe symptomatic TR demonstrated by echocardiography, NYHA Class III or IV, LVEF of >30% and suitable anatomic criteria by computed tomography. We excluded patients with right ventricular failure (TAPSE less than 1.3 cm), systolic pulmonary artery pressure of more than 65 mmHg by Doppler echo, liver cirrhosis Child-Pugh C, and those with congenital structural heart disease. Aside from these criteria, a preprocedural CT scan assessment is mandatory to ensure the venous anatomy is suitable for delivery of the device to the SVC and IVC. So far, there are still limited data on the long-term outcome of such devices. Our case also highlights the value of telemedicine for proctorship in structural heart intervention amidst the COVID-19 pandemic7.
Limitations
An initial computed tomography (CT) scan is mandatory to ensure the anatomy is suitable for delivery of the system. No follow-up CT was done, as follow-up echocardiograms were deemed adequate for assessment. Elective intubation with general anaesthesia was performed to ensure a more controlled environment, however it is not necessary. Echocardiography guidance is primarily required for the IVC implantation to be performed with the proper care as well as to avoid either a paravalvular leak or hepatic vein obstruction by ensuring that no more than 20 mm of the IVC stent protrudes into the right atrium. Also, transthoracic echocardiography would be sufficient in centres with more experience.
Conclusions
Bicaval valve implantation with the TricValve is a safe and effective non-surgical option for patients with symptomatic severe tricuspid regurgitation. Early intervention in such cases is crucial, and this is now possible with the availability of the TricValve.
Impact on daily practice
Bicaval valve implantation via a transcatheter approach was initially intended for compassionate use. However, it can play a pivotal role as an elective procedure for selected patients who are showing signs of worsening heart failure due to severe tricuspid regurgitation (TR), particularly atrial functional TR for which treatment options are limited. This case illustrates the importance of early intervention in such patients for whom prognosis is poor due to irreversible RV dysfunction and surgical treatment that carries high perioperative mortality.
Acknowledgements
We would like to acknowledge the cardiac anaesthesia/intensive team led by Dato’ Dr N. Thiru Kumar A/L A. Namasiwayam and Dato’ Dr Suneta Sulaiman as well as the cardiothoracic team led by Prof. Dato’ Dr Mohamed Ezani Md Taib and Dato’ Dr Mohd Nazeri Nordin for their support and help in making this endeavour successful. A special thanks to our dedicated senior echocardiographers Mohammed Dzaqqee Yameen Bin Zainal and Mr Deventhiran Permal. We would also like to extend our gratitude to the proctors Dr Ignacio J. Amat Santos from Spain and Dr Katharina Kiss for their guidance.
Conflict of interest statement
The authors have no conflicts of interest to declare.
