Introduction
The advent of coronary revascularisation in the early 20th century and the arduous journey of its refinement have greatly influenced and transformed the landscape of coronary artery disease management123. This is undeniably contributed to by the concurrent deepening of our understanding of the pathophysiology of the disease and its progression. In addition to medical therapy, the two principal options for revascularisation would be surgical correction with coronary artery bypass graft (CABG) and percutaneous coronary intervention (PCI).
CABG, an open surgical procedure, aims to achieve complete revascularisation where a graft vessel is anastomosed to existing coronary vasculature, bypassing atherosclerotic occlusions that reduce luminal flow and, hence, re-establishing coronary blood flow to the myocardium. A decade and a half after the discovery of CABG, the pinnacle of revascularisation, the emergence of an alternative procedure, namely PCI, came about. In contrast to CABG, PCI is a minimally invasive procedure involving the insertion of a device percutaneously through a peripheral arterial site and threaded into the location of the stenosis or occlusion, where it is inflated. It confers different degrees of disease intervention and a myriad of choices of devices, from balloon angioplasty to bare metal stents and, most recently, the use of drug-eluting stents2. While some innovative stents have been withdrawn from the market, drug-eluting stents have become the current standard of practice456. Although CABG and PCI fulfil similar roles in achieving the end result of revascularisation, both show varying efficacy in different patient groups.
Clinical outcomes of PCI and CABG in the contemporary era
Landmark randomised controlled trials and meta-analyses have established that, depending on the anatomical complexity, the extent of coronary artery lesion involvement, and pre-existing patient comorbidities, each revascularisation method has its own relative merits in providing for an individual instead of casting a blanket treatment on a patient789. It has been generally accepted, based on randomised trials, that CABG is the recognised gold standard of choice for patients with diffuse multivessel coronary artery disease and/or diabetes mellitus with a high anatomical complexity score7.
Revascularisation effectively addresses obstructions in the coronary vasculature that have already transpired and relieves cardiac ischaemia. However, as described above, we are beginning to recognise the importance of periprocedural patient optimisation, where addressing certain premorbid risk factors (such as hyperlipidaemia and diabetes mellitus) contributes to an improved procedural outcome101112. One such risk factor, which has not been widely researched, is obstructive sleep apnoea (OSA)1314. In the past decades, untreated OSA has been shown to be an independent predictor of adverse cardiovascular outcomes in patients undergoing coronary revascularisation.
OSA − pathophysiology, diagnosis, and prevalence
OSA is a chronic sleep disorder characterised by recurrent cyclical episodes of upper airway collapse causing cessation of breathing (apnoea) or a reduction in airflow (hypopnoea) during complete and partial obstruction, respectively13. This commonly presents as snoring, choking, or absence of breathing during sleep and may manifest in the form of oxygen desaturations, arousals, and fragmented sleep.
The pathophysiology of OSA is multifaceted, mainly stemming from a sleep-induced dimensional reduction of the upper airway. Pharyngeal function and patency are controlled by static and dynamic components, namely morphological skeletal and soft tissue framework, as well as the synchronised neuromuscular tone of more than 20 muscles. Craniofacial features, fatty tissue depositions, and inflammatory soft tissue oedema in the neck, which can all reduce the airway diameter, and the rostral shift of fluid to the neck during sleep are some examples of anatomical impairments. Any alterations in sleep-related physiological phenomena would result in variable pharyngeal collapsibility, causing a disruption in respiratory mechanics. A clinical measure used to quantify this would be the passive critical occlusion pressure, which is the endopharyngeal pressure associated with upper airway collapse. In addition, studies have shown that other physiological factors associated with OSA include the loop gain (ventilatory control instability) and arousal threshold (sleep-wake instability).
Statistics have shown that approximately 70% of patients have more than one non-anatomical phenotype, with each phenotype having a varying contribution to the degree and progression of the disease, giving rise to a wide potential for disease severity. Hence, qualitative phenotyping allows us to characterise the different pathophysiological traits in OSA patients and, potentially, improve the identification of more patients for diagnosis. A combination of both subjective and objective investigations is necessary for the holistic evaluation and diagnosis of patients. Subjective assessments, such as the Berlin Questionnaire and the Epworth Sleepiness Scale Questionnaire, allow clinicians to qualify self-reported nocturnal and daytime symptoms of OSA, taking into consideration individual anthropometric characteristics.
Different methods to diagnose OSA are summarised in Table 1; the level 1 overnight in-laboratory polysomnography, conducted and supervised by a certified sleep technologist, remains the diagnostic gold standard. During the polysomnography, the following parameters are recorded: airflow (nasal cannula and thermistor), respiratory movements (respiratory inductance plethysmography), oxygen saturation (as measured by pulse oximetry), snoring episodes, electrocardiography, and body position. OSA was diagnosed on the basis of the apnoea-hypopnoea index (AHI), quantified as the total number of apnoeas or hypopnoeas recorded per hour of sleep. According to the American Academy of Sleep Medicine (AASM) guideline15, apnoea is defined as a ≥90% decrease in airflow from baseline for at least 10 seconds, and hypopnoea is defined as a ≥30% decrease in airflow from baseline for ≥10 seconds, associated with either an oxygen desaturation of ≥3% (some centres use ≥4%) and/or arousal. Diagnosis of OSA entails the presence of daytime or nocturnal symptoms alongside an AHI ≥5 or, in the absence of symptoms, AHI ≥15.
There is a unanimous tacit consensus among physicians worldwide that OSA is an underdiagnosed disease entity. Exacerbating this state of the affair is the global prevalence of OSA, with an estimated 1 billion people affected and with prevalence exceeding 50% in several countries16. According to The Lancet 201916, 936 million (95% confidence interval [CI]: 903-970) adults (men and women) aged 30-69 years old have mild to severe OSA, and 425 million (399-450) adults aged 30-69 years old have moderate to severe OSA, globally, based on AASM 2012 diagnostic criteria and the AHI threshold values of ≥5 events per hour and ≥15 events per hour, for symptomatic and non-symptomatic disease, respectively. A community-based study in Singapore17 exemplifies this, where the weighted estimates of the population prevalence of moderate to severe OSA and sleep apnoea syndrome were 30.5% and 18.1%, respectively17. Essentially, 91.0% of subjects with an AHI ≥15 events per hour were previously undiagnosed17.
It is well established that there is a strong predominance of OSA in men and obese and middle-aged patients, with additional risk factors encompassing family history, race, and ethnicity. More importantly, OSA is increasingly becoming a recognised cause of increased cardiovascular risk18. It has been surmised that this may potentially be mediated, in part, by its strong association with cardiovascular complications such as hypertension, atrial fibrillation, coronary artery disease, congestive heart failure, stroke, diabetes, and metabolic syndrome14.
Table 1. American Academy of Sleep Medicine classification of sleep apnoea evaluation.
| Level | Level I. Standard polysomnography | Level II. Comprehensive portable polysomnography | Level III. Modified portable sleep apnoea testing | Level IV. Continuous (single or dual) bioparameter recording |
|---|---|---|---|---|
| Minimum recording channels | EEG, EOG, chin EMG, ECG, airflow, respiratory effort, and oxygen saturation. | Same as for Level I except heart rate instead of ECG is acceptable. | Recording of ventilation (at least 2 channels of respiratory movement, or respiratory movement and airflow), ECG or heart rate and oxygen saturation. | Only 1 or 2 physiological variables need to be recorded. |
| Other characteristics | Body position must be documented or objectively measured. Leg movement recording (EMG or motion sensor) is desirable but optional. | |||
| Personnel and ability to intervene | Trained personnel must be in constant attendance and able to intervene. | Personnel are needed for preparation. Ability to intervene is not required for all studies. | Personnel are needed for preparation. Ability to intervene is not required for all studies. | Personnel are needed for preparation. Ability to intervene is not required for all studies. |
| ECG: electrocardiogram; EEG: electroencephalogram; EMG: electromyogram; EOG: electro-oculogram | ||||
OSA − a novel cardiovascular risk factor
As detailed above, OSA is a complex and heterogeneous disease characterised by multiple underlying mechanisms. The immediate effects of repeated attempts to inspire against an obstructed upper airway include a drop in intrathoracic pressure, cortical arousal from sleep, hypoxia, and sympathetic activation. Each of these, in turn, gives rise to the adverse cardiovascular outcomes expounded below.
Exposure to prolonged negative intrathoracic pressure results in decreased left ventricular filling and increased afterload, ultimately reducing stroke volume. Furthermore, OSA causes marked, repeated BP elevation and tachycardia secondary to sympathetic nerve hyperactivity19. The sympathetic nervous system is further augmented by decreased stroke volume and the suppression of sympathetic inhibitory effects of lung stretch receptors during apnoea. Sleep arousal and respiratory events during OSA also result in peripheral vasoconstriction, the release of catecholamines and a reduction in parasympathetic modulation of the heart, resulting in elevated blood pressure (BP) during the night.
The net effect of increased left ventricular afterload, tachycardia, and BP elevation leads to myocardial oxygen supply-demand mismatch, ultimately resulting in (i) acute predisposition to cardiac ischaemia and arrhythmias and (ii) chronic predisposition to left atrial enlargement and left ventricular hypertrophy. This is aggravated by recurrent upper airway collapse which results in increased oxidative stress and reduced production of endothelium-dependent vasodilator substances20, such as nitric oxide, contributing to vascular dysfunction and systemic inflammation. These processes ultimately lead to myocardial fibrosis and left ventricular diastolic dysfunction.
OSA is strongly associated with hypertension, and a dose-response relationship exists between the severity of OSA and the degree of hypertension. In addition, OSA plays an essential role in resistant hypertension and may mediate the association with cardiovascular disease. The perturbations caused by OSA not only provide a clear mechanistic link to cardiovascular disease but also to coronary revascularisation outcomes.
OSA and coronary revascularisation outcomes
OSA and PCI
There is a lack of data from extensive cohort studies examining the prognostic significance of OSA in patients treated with PCI. In the pre-drug-eluting stent era, single-centre studies found OSA to be a predictor of restenosis and target vessel revascularisation [21,22]. A non-randomised study suggested that patients who received treatment for OSA had reduced cardiac mortality 5 years after PCI compared with those who declined treatment [23]. OSA has been linked with coronary plaque burden and an increased risk of cardiovascular disease [24]; however, there were few studies on the prognostic effect of OSA in patients undergoing PCI before the Sleep and Stent Study (ClinicalTrials.gov: NCT02215317).
The Sleep and Stent Study is the largest multinational cohort study examining the effects of OSA on post-PCI cardiovascular outcomes to date [25]. The Sleep and Stent Study was a prospective, multicentre study designed to assess the association between OSA and cardiovascular outcomes in patients treated with PCI. Overall, a total of 1,748 patients who had undergone a successful PCI in at least one coronary artery were enrolled in the study. The prespecified primary endpoint was a major adverse cardiac event (MACE): a composite of cardiovascular mortality, non-fatal myocardial infarction, non-fatal stroke, and unplanned revascularisation. In total, 1,311 patients completed a portable sleep study within 7 days of their PCI procedure. OSA, defined as an AHI ≥15 events per hour, was diagnosed in 45.3% of the patient cohort (n=594). Patients with OSA were older, more likely to be male, had a higher BMI, and had a higher prevalence of hypertension and diabetes mellitus than those without OSA. During the median follow-up of 1.9 years, a MACE occurred in 141 patients (3-year cumulative incidence estimate: 16.4%), including cardiovascular death in 24 patients. The incidence of MACE was higher in patients with OSA than in those without (3-year estimate: 18.9% vs 14.0%; p=0.001) (Figure 1). Cox regression analysis showed that OSA was an independent predictor of MACE (hazard ratio [HR] 1.57, 95% CI: 1.10-2.24; p=0.013).
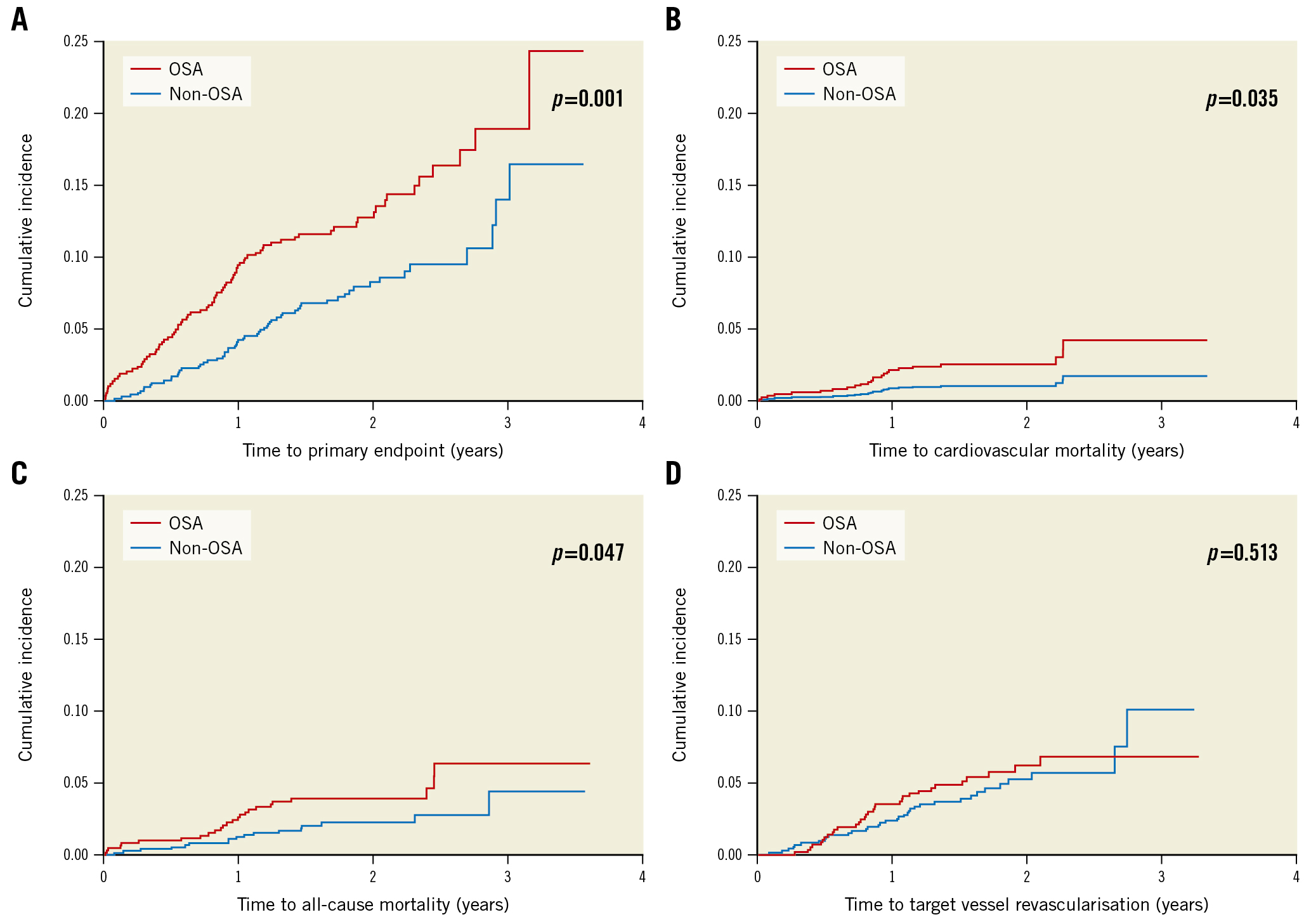
Figure 1. Kaplan-Meier cumulative incidence curves of study endpoints. Cumulative incidence of (A) the primary endpoint, (B) cardiovascular mortality, (C) all-cause mortality, and (D) target vessel revascularisation. The primary endpoint comprises cardiovascular mortality, non-fatal myocardial infarction, non-fatal stroke, and unplanned revascularisation. OSA: obstructive sleep apnoea
OSA and CABG
The Undiagnosed Sleep Apnea and Bypass OperaTion (SABOT) study was designed to evaluate the association between OSA and MACE in patients undergoing non-emergent CABG [26]. Patients with multivessel coronary artery disease are often referred for CABG, and it remains unknown if OSA is a risk marker. Patients residing in Singapore and who were referred to a tertiary cardiac centre for non-emergent CABG were eligible. The recruited participants underwent an overnight sleep study using a wearable diagnostic device before undergoing CABG. Among the 1,007 patients who completed the study, OSA (defined as an AHI ≥15 events per hour) was diagnosed in 513 patients (50.9%). Over a mean follow-up period of 2 years, 124 patients experienced the 4-component MACE (2-year cumulative incidence estimate: 11.3%). There were a total of 33 cardiac deaths (2.5%), 42 non-fatal myocardial infarctions (3.7%), 50 non-fatal strokes (4.9%), and 36 unplanned revascularisations (3.2%). The crude incidence of MACE was higher in the OSA group than in the non-OSA group (2-year estimate: 14.7% vs 7.8%; p=0.002) (Figure 2). Similarly, the crude incidence rates of all-cause mortality, cardiovascular mortality, sudden cardiac death or resuscitated cardiac arrest, and hospitalisation for heart failure were also higher in the OSA group (Figure 3). OSA was a predictor of MACE in unadjusted Cox regression analysis (HR 1.69), and the association remained statistically significant after adjustment for the effects of confounding variables (adjusted HR 1.57).
An auxiliary study evaluated the association between OSA and total heart failure hospitalisation (HFH) after CABG. Approximately 10% of the 1,007 recruited patients had at least one episode of HFH [27,28]. At a mean follow-up of 3.3 years, a subgroup of 37% of patients had recurrent events that accounted for two-thirds of the total 179 HFH events. Using four robust statistical methods (Poisson, negative binomial, Andersen-Gill, and joint frailty models), we found that patients with OSA had a 1.6- to 1.8-fold increased risk of recurrent HFH, even after adjustment for differences in baseline demographic and clinical characteristics.
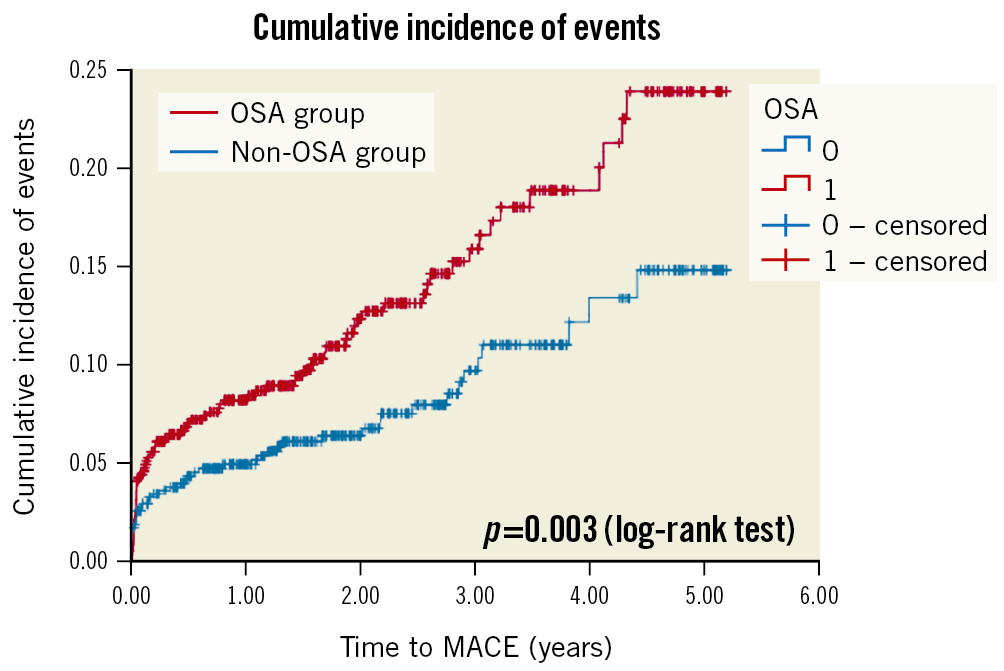
Figure 2. Cumulative incidence of the composite primary endpoint of major adverse cardiac events. Kaplan-Meier plot showing the cumulative incidence of major adverse cardiac events in patients with OSA (in red) and in patients without OSA (in blue). MACE: major cardiac adverse events; OSA: obstructive sleep apnoea
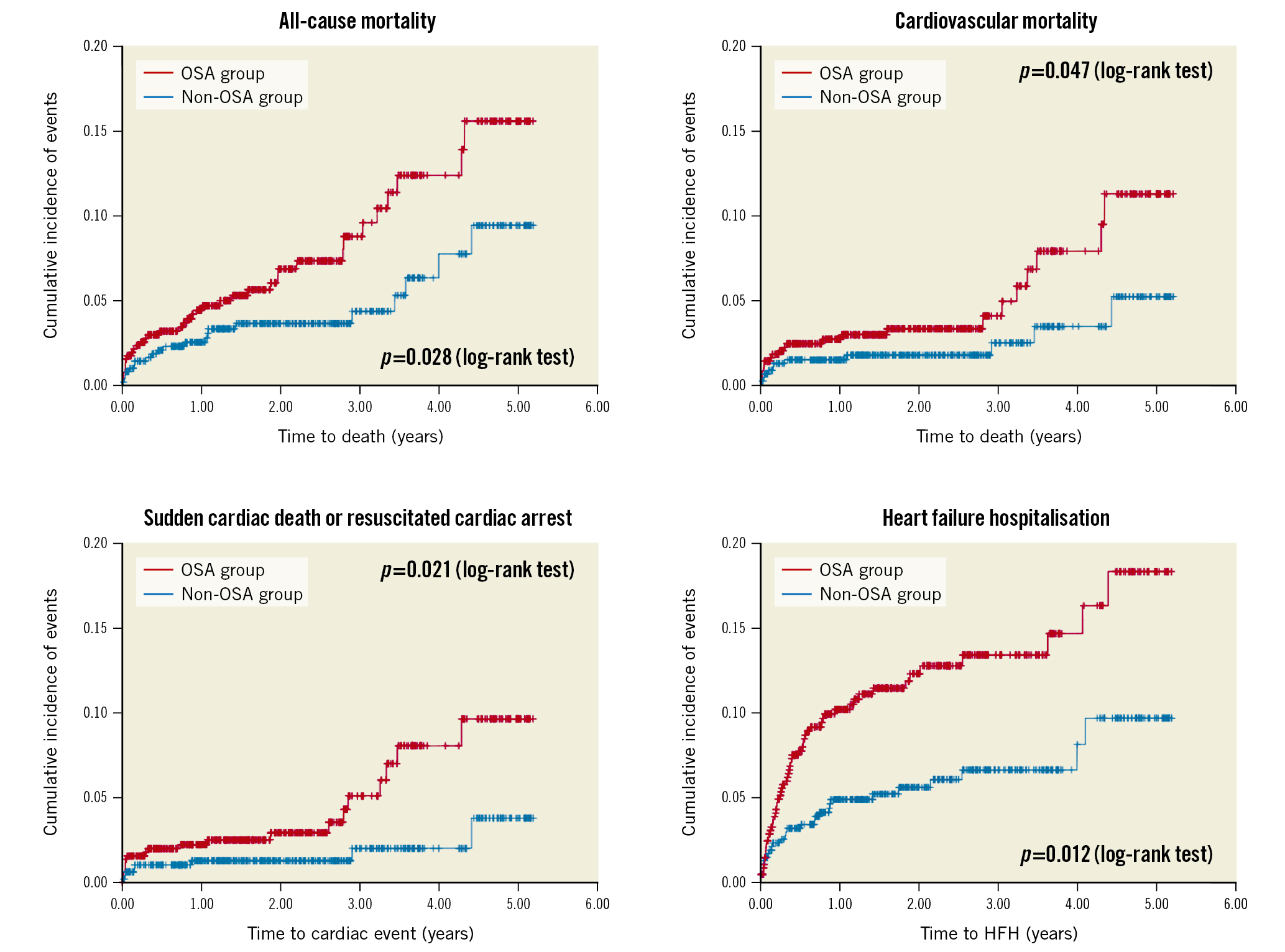
Figure 3. Cumulative incidence of secondary endpoints. Kaplan-Meier plots showing the cumulative incidences of all-cause mortality (A), cardiovascular mortality (B), sudden cardiac death or resuscitated cardiac arrest (C) and hospitalisation for heart failure (D), respectively, in patients with OSA (in red) and in patients without OSA (in blue). HFH: heart failure hospitalisation; OSA: obstructive sleep apnoea
Treatment of OSA to improve cardiovascular outcomes
RICCADSA, SAVE, and ISAACC
Despite ample evidence of the association between continuous positive airway pressure (CPAP) treatment and improvement in blood pressure and endothelial function2930, this is not sufficient to incorporate systematic OSA screening and treatment into the cardiovascular guidelines without supportive data from randomised clinical trials. In the past decade, three randomised controlled trials have been conducted to explore the potential benefits of CPAP in cardiovascular outcomes. These are summarised in Table 2. The first of these is the Randomized Intervention With CPAP in Coronary Artery Disease and Sleep Apnea – RICCADSA Trial31. In this single-centre trial, 244 patients with newly revascularised coronary artery disease and OSA (AHI ≥15 events per hour) were randomised to CPAP or usual care in a 1:1 ratio. The primary endpoint was a composite of repeat coronary revascularisation, myocardial infarction, stroke, or cardiovascular mortality. Over a median follow-up of 57 months, the incidence of the primary endpoint was 18.1% (CPAP group) versus 22.1% (usual care group) (p=0.449).
The Continuous Positive Airway Pressure Treatment of Obstructive Sleep Apnea to Prevent Cardiovascular Disease (SAVE) Study was a multicentre randomised trial and is the largest of the three listed trials32. In contrast to most trials, where OSA was diagnosed based on the AHI, OSA was diagnosed based on an oxygen desaturation index ≥12 events per hour in the SAVE Study. Patients with stable coronary artery disease or cerebrovascular disease (n=2,717) and OSA were randomised to CPAP (n=1,346) or usual care (n=1,341). The primary outcome was a composite of death from cardiovascular causes, myocardial infarction, stroke or hospitalisation for unstable angina, and heart failure or transient ischaemic attack. Over a mean follow-up of 3.7 years, the incidence of the primary endpoint was 17.0% (CPAP group) versus 15.4% (usual care group) (p=0.34).
The CPAP in Patients With Acute Coronary Syndrome and OSA (ISAACC) Study was a multicentre randomised trial of patients with acute coronary syndrome33. All patients underwent respiratory polygraphy during the acute phase, and patients with OSA were randomised to CPAP (n=633) or usual care (n=631). Over a median follow-up of 3.4 years, the incidence of the primary endpoint (a composite of cardiovascular death or non-fatal events [acute myocardial infarction, non-fatal stroke, hospital admission for heart failure, and new hospitalisations for unstable angina or transient ischaemic attack]) was 16% in the CPAP group versus 17% in the usual care group (p=0.40). The major limitation of the ISAACC trial is the timing of the sleep study. A recent study has demonstrated that after acute coronary syndrome, concurrent changes occurred in the AHI, left ventricular ejection fraction, and left ventricular end-systolic volume. Of the patients diagnosed with OSA at the acute phase, resolution of OSA was seen in 48% at 6 months34.
None of the three trials showed a clear benefit of OSA therapy using CPAP in improving cardiovascular outcomes. However, further data analysis revealed limitations in the design and execution of the studies. The adherence to CPAP in these trials was low. Indeed, all of these trials reported an average CPAP adherence of <4 hours per night, which is below the minimum (≥4 hours) needed to derive benefits from CPAP35. Notably, these trials recruited patients who had developed cardiovascular events. Such patients differ from younger patients, whose CPAP may confer a benefit in the primary prevention of cardiovascular disease, whereby they may be more likely to adhere to CPAP use.
Table 2. Summary of the three randomised controlled trials on the effects of CPAP on cardiovascular events.
| Trial | Single- or multicentre study | Number of patients recruited | Study period | Key inclusion criteria | Key exclusion criteria | Definition of OSA | Mean ESS* (CPAP vs usual care) | Average CPAP adherence (h/night) | Percentage of patients with CPAP adherence ≥4 h/night) |
|---|---|---|---|---|---|---|---|---|---|
| RICCADSA | Single-centre | 244 | 2005-13 | Adult patients with CAD who had undergone PCI or CABG in, the previous 6 months | Patients with existing OSA, daytime sleepiness (ESS >10), and predominantly central apnoeas with Cheyne-Stokes respiration | AHI >15 events/h | NA | NA | NA |
| SAVE | Multicentre | 2,717 | 2008-16 | Adults between 45 and 75 years of age who had OSA and stable coronary or cerebrovascular disease | Severe daytime sleepiness (ESS >15) or were considered to have an increased risk of an accident from falling asleep, very severe hypoxaemia, or Cheyne-Stokes respiration | ODI** ≥12 events/h | 7.3±3.6 vs 7.5±3.6 | 3.3 h/night | 42% |
| ISAACC | Multicentre | 2,551 | 2011-18 | Aged ≥18 years, hospitalised for ACS | Previous treatment with CPAP for OSA, inability to complete questionnaires, known sleep disorder, >50% central apnoeas or the presence of Cheyne-Stokes respiration, and daytime sleepiness (ESS >10) | AHI ≥15 events/h | 5.4±2.5 vs 5.3±2.5 | 2.8 h/night | 38% |
| *scores range from 0 to 24, with higher scores indicating greater severity. Daytime sleepiness generally defined as ESS >10. **the number of times per hour during the oximetry recording that the blood oxygen saturation level drops by ≥4 percentage points from baseline. ACS: acute coronary syndrome; AHI: apnoea-hypopnoea index; CABG: coronary artery bypass grafting; CAD: coronary artery disease; CPAP: continuous positive airway pressure; ESS: Epworth Sleepiness Scale; ISAACC: CPAP in Patients With Acute Coronary Syndrome and OSA; NA: not available; ODI: oxygen desaturation index; OSA: obstructive sleep apnoea; PCI: percutaneous coronary intervention; RICCADSA: Randomized Intervention With CPAP in Coronary Artery Disease and Sleep Apnea; SAVE: Continuous Positive Airway Pressure Treatment of Obstructive Sleep Apnea to Prevent Cardiovascular Disease | |||||||||
Low CPAP adherence in clinical trials and its implications
All the research participants in the aforementioned trials presented with cardiovascular disease. These participants were unable to tolerate the CPAP over the duration of the trials to achieve clinically meaningful benefits, possibly due to excessive daytime sleepiness or drug (angiotensin-converting enzyme inhibitor)-induced airway hyperresponsiveness36. In the RICCADSA Trial, 38% of the participants in the CPAP group stopped using the device within the first year. The adjusted on-treatment analysis showed a cardiovascular risk reduction in those who used CPAP for ≥4 versus <4 hours per night (p=0.026), suggesting that the low CPAP adherence may have contributed to the overall negative results. In the SAVE trial, despite the initial run-in period with sham CPAP achieving an average usage of 5.2 hours per night, CPAP usage declined over the first year to 3.5±2.4 hours per night and was only 3.3±2.3 hours per night at the final follow-up. Moreover, only 42% of participants in the CPAP group achieved the conventional criteria for good adherence (≥4 hours per night). The propensity score-matched analyses showed that the patients adhering to CPAP therapy had a lower risk of stroke (p=0.05) and the composite endpoint of cerebral events (p=0.02) than those in the usual care group. Similarly, adherence to CPAP was extremely low in the ISAACC Study. Indeed, 1 year after starting CPAP, the average adherence was only 2.8±2.6 hours per night, with only 36% of the patients in the CPAP group achieving ≥4 hours per night. A propensity score analysis comparing patients who achieved “good adherence” with those receiving usual care showed an HR of 0.80, favouring the CPAP group37.
It has long been recognised that some patients diagnosed with OSA are not receptive to CPAP, despite it being a guideline-mandated first-line treatment for OSA that improves snoring and daytime sleepiness. Using recent data from 789,260 patients initiated on CPAP in the US Centers for Medicare & Medicaid Services database, the overall adherence rate (≥4 hours of use on 70% of nights over a consecutive 30-day period) in the first 90 days was only 72.6%38.
Despite the introduction and testing of many interventional measures, these interventions have had limited success, and this treatment modality continues to be plagued by a lack of adherence. Indeed, overall non-adherence remains consistent at 30%-40%, especially in health systems where the cost of CPAP is not reimbursable3940.
Conclusions
OSA is a prevalent, chronic sleep disorder that affects a patient’s quality of life. In the past decades, there has been emerging evidence that OSA is an independent predictor of adverse cardiovascular outcomes in patients undergoing coronary revascularisation. Affected individuals had a 50% higher risk of experiencing adverse events if the OSA was untreated. CPAP, with positive airway pressure applied through a nasal or oronasal interface to splint the upper airway open, is the mainstay of therapy for OSA. CPAP is effective in alleviating OSA-associated sleepiness. Moreover, epidemiological data have demonstrated that patients with OSA who use CPAP have a lower risk of fatal and non-fatal cardiovascular events than non-users. Similarly, randomised trials have shown the benefits of CPAP in improving systolic blood pressure, inflammation, and endothelial function. However, due to poor CPAP adherence, randomised controlled trials have failed to verify the benefits of CPAP in reducing cardiovascular events. For now, systematic screening for OSA in patients undergoing coronary revascularisation is not indicated. Although, screening for and treatment of OSA is still indicated if the patients have reported excessive daytime sleepiness and/or suboptimally controlled hypertension.
Funding
This study was supported by the Clinician Scientist Award (Senior Investigator category) from the National Medical Research Council (Singapore).
Conflict of interest statement
The authors have no conflicts of interest to declare.

