Introduction
The prevalence of cardiovascular diseases among younger adults is increasing1. Adults who experience acute coronary syndrome (ACS) at a younger age are reported to have a different pathophysiology compared to older adult patients1. Optical coherence tomography (OCT), a high-resolution intracoronary imaging technique, facilitates a thorough assessment of plaque morphology in ACS patients2. The major mechanisms for ST-segment elevation myocardial infarction (STEMI) include plaque rupture, plaque erosion, and calcified nodules – all of which may be readily visualised by intravascular imaging23. Additionally, OCT can evaluate the healing process of plaque rupture or erosion along with thrombus detection, which may help in understanding the underlying disease mechanism45.
There are conflicting data on the morphological characteristics of culprit plaques in different age groups. One European multicentre OCT registry has shown that young patients (age ≤50 years) with STEMI are more likely to have a higher prevalence of culprit plaque rupture, a thinner cap, and fewer fibrotic or fibrocalcific components as compared to the elderly6. On the contrary, a Chinese OCT registry7 suggested that younger patients (age <50 years) with STEMI had more plaque erosions and fewer thin-cap fibroatheromas (TCFAs).
The present study aimed to investigate the morphological characteristics of culprit plaque and thrombus in very young adult (≤35 years) versus older adult STEMI patients (>60 years).
Methods
STUDY DESIGN AND POPULATION
This was a prospective, single-centre, investigator-initiated study using OCT to examine culprit lesion morphology in very young adult patients (≤35 years) compared with older adult patients (>60 years) with STEMI (Central illustration). The diagnosis of STEMI was based on the Fourth Universal Definition of Myocardial Infarction (MI)8. Patients with STEMI who underwent thrombolysis within 48 hours from symptom onset at peripheral hospitals and were then transferred to a tertiary care centre for further evaluation and treatment were screened, consecutively. Patients with left ventricular ejection fraction ≥35% who agreed to comply with all specified study requirements were included. Exclusion criteria were acute heart failure or shock, renal failure, prior coronary bypass surgery, allergy to contrast media, life expectancy of <1 year, and pregnancy. Angiographic exclusion criteria were left main disease, chronic total occlusion, tortuous or calcified vessels through which OCT contrast medium was not expected to pass, or stent thrombosis. At the beginning of the study, only very young patients (≤35 years) were enrolled. Culprit lesions were identified through localising findings from electrocardiograms, echocardiograms, and coronary angiograms.
The present study was approved by the institutional ethics committee and was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice and local regulations. All enrolled patients provided written informed consent for the study.
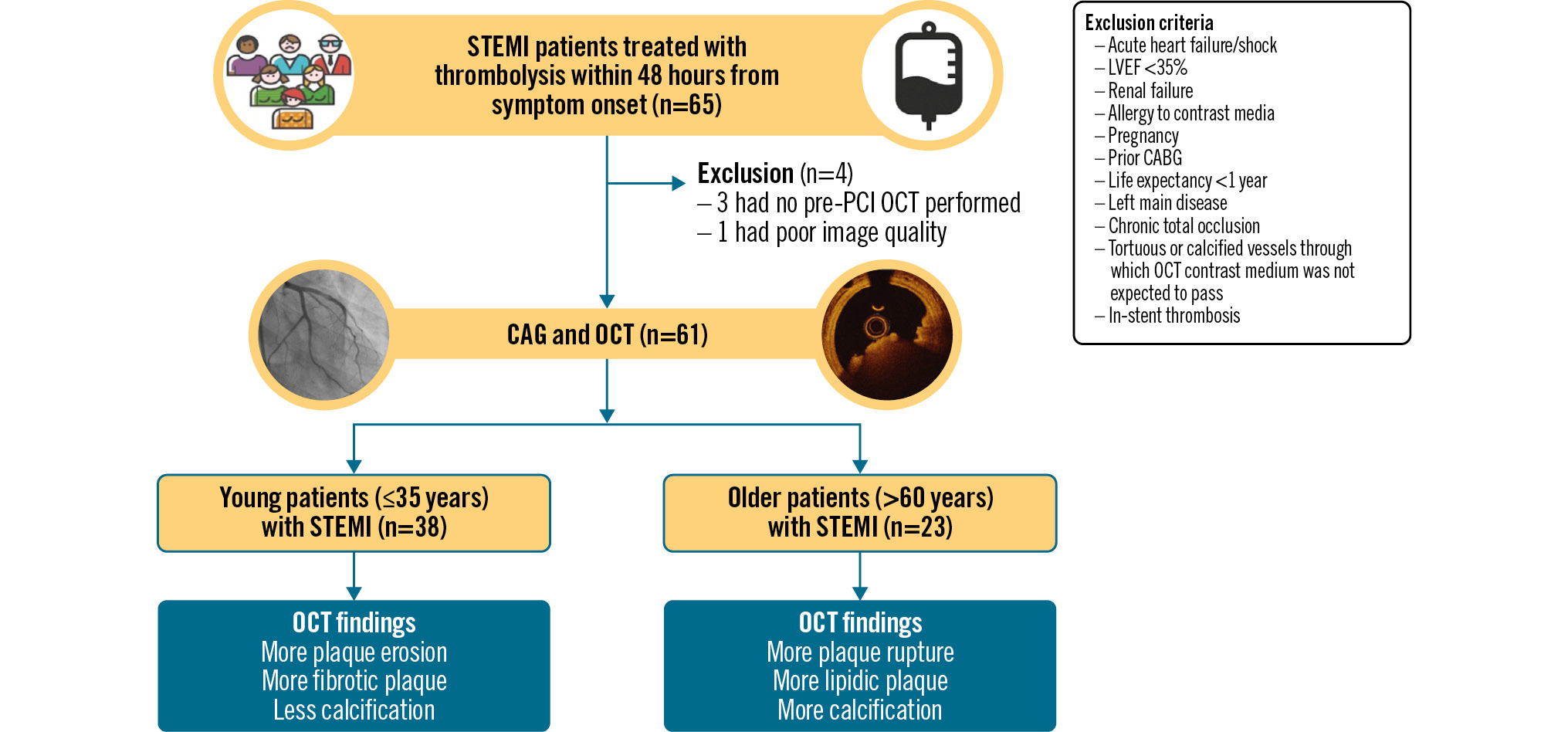
Central illustration. Study flowchart. CABG: coronary artery bypass grafting; CAG: coronary angiography; LVEF: left ventricular ejection fraction; OCT: optical coherence tomography; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction
CORONARY ANGIOGRAPHY ANALYSIS
Quantitative coronary angiographic analysis was performed using QAngio XA, version 7.2 (Medis Medical Imaging Systems) by independent cardiologists blinded to the clinical data9.
OCT IMAGE ACQUISITION AND ANALYSIS
All enrolled patients underwent coronary angiography followed by OCT using standard techniques. After administration of intracoronary nitroglycerine (200 μg), OCT images were acquired using a frequency domain OCT system (ILUMIEN OPTIS) and a Dragonfly OPTIS OCT catheter (both Abbott). A 1.5 mm compliant balloon was inflated at nominal pressure to predilate the lesion if the OCT catheter was unable to pass beyond the lesion. Automated pullback was triggered with intracoronary contrast injection (3-4 ml/s, 12-14 ml total) with a motorised pullback speed of up to 25 mm/s and a frame rate of 100/s. All OCT images were analysed offline using proprietary software (OPTIS Offline Review Workstation software, version E.4.1 [Abbott]) by an OCT core laboratory blinded to the clinical data (Cardiovascular Research Foundation, New York, NY, USA).
OCT morphologies were classified according to established OCT reporting standards1011. Briefly, plaque rupture was defined as disruption of a fibrous cap overlaying lipidic plaque (Figure 1A). Plaque erosion was defined as the presence of an intact fibrous cap with attached thrombus, irregularity of the lumen of the culprit lesion in the absence of thrombus, or lesions with underlying plaque attenuated by thrombus without superficial lipid or calcium immediately proximal or distal to the site of thrombus (Figure 1B). A calcified nodule was defined as an accumulation of small calcium fragments protruding into the lumen with strong attenuation (Figure 1C). Thrombus was defined as an irregular intraluminal mass (>250 μm) which was either attached to the vessel wall or free-floating in the lumen and was subclassified into red (high backscatter with high attenuation) or white thrombus (low backscatter with low attenuation). Additionally, thrombus age was subclassified into acute (intraluminal thrombus with surface irregularity) or subacute (mostly comprised of mural thrombus with a smooth surface with some findings of acute thrombus) (Figure 1D). If the surface tissue of the culprit lesion had a smooth layer demarcated with underlying plaque, it was considered as late thrombus and/or healed plaque (Figure 1E)512. Multiple intraluminal communicating channels separated by septa (honeycomb pattern) were considered to represent late thrombus (Figure 1F). If the culprit lesion had evidence of ruptured cavity (i.e., intraplaque haemorrhage defined as a low intensity region without attenuation), overlaying late thrombus and/or healed plaque adjacent to lipidic plaque, it was categorised as plaque rupture121314. If the culprit lesion had only late thrombus and/or healed plaque overlaying fibrous plaque without adjacent lipidic plaque, it was categorised as plaque erosion5. When none of the above findings were observed, it was considered indeterminate. Lipidic plaque was defined as a region with strong signal attenuation with poorly delineated borders that was covered by a fibrous cap. Fibrous cap thickness was measured three times at the thinnest part, and the average value was calculated. TCFA was defined as lipidic plaque >90° with a fibrous cap thickness <65 μm. Fibrous plaque was homogeneous plaque with high backscatter. Calcified plaque was a signal-poor or heterogeneous region with a sharply delineated border.
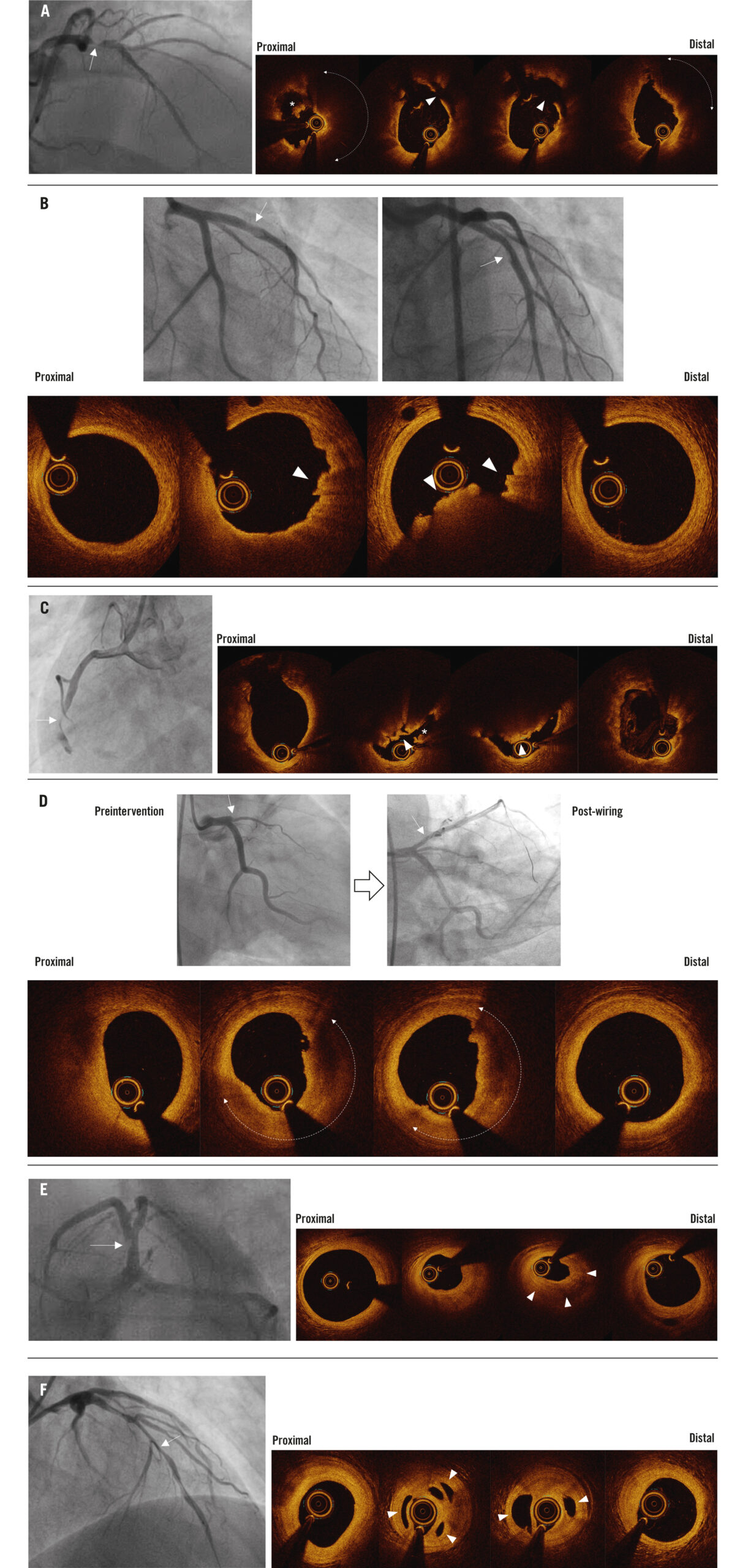
Figure 1. Representative cases. For each case, the first image(s) is the coronary angiogram (with white arrows indicating the location of the optical coherence tomography [OCT] images), and the subsequent images are from OCT. A) Plaque rupture with acute thrombus. This coronary angiogram from a 68-year-old male with ST-segment elevation myocardial infarction (STEMI) shows severe luminal narrowing in the proximal left anterior descending artery (LAD). The OCT images show plaque rupture (arrowheads) with acute thrombus formation (asterisk) overlying lipidic plaque (dotted line with double arrowheads). B) Plaque erosion with acute thrombus. This coronary angiogram from a young 26-year-old male with STEMI shows haziness in the proximal LAD. The OCT images show plaque erosion with acute thrombus formation (arrowheads). C) Eruptive calcified nodule with acute thrombus. This coronary angiogram from a 68-year-old male with STEMI shows the presence of thrombus with luminal narrowing in the proximal and mid right coronary artery (RCA). The OCT images show an eruptive calcified nodule with overlaying acute thrombus (asterisk). D) Plaque erosion with subacute thrombus. This preinterventional coronary angiogram from a 35-year-old male with STEMI shows a total occlusion in the proximal LAD. After wiring, the lesion showed mild narrowing. The OCT images show plaque erosion with subacute thrombus formation (dotted lines with double arrowheads). E) Plaque erosion with late thrombus and/or healed plaque. This coronary angiogram from a 28-year-old male with STEMI shows a filling defect in the proximal LAD. The OCT images show plaque erosion with late thrombus and/or healed plaque formation (arrowheads). F) Plaque erosion with honeycomb pattern of late thrombus. This coronary angiogram from a 35-year-old male with STEMI shows diffuse narrowing in the LAD. The OCT images show plaque erosion with multiple intraluminal communicating channels separated by septa (arrowheads), which are considered to represent recanalised late thrombus.
STATISTICAL ANALYSIS
Normally distributed continuous data are expressed as mean±standard deviation and were compared using an unpaired Student’s t-test. Non-normally distributed continuous data are shown as median (first quartile, third quartile) and were compared using the Mann-Whitney U test. Categorical data are represented as proportions and were compared using the chi-square or Fisher’s exact test, as appropriate. A 2-sided p-value<0.05 was considered statistically significant. SPSS Statistics, version 24.0 (IBM) and Prism, version 8.0.0 (GraphPad Software) software were used for statistical analysis.
Results
Between January 2020 and January 2022, a total of 65 STEMI patients were enrolled. After exclusion of 4 patients with no pre-stent OCT or poor-quality OCT images (2 in each group), 61 patients were included in the final analysis. The time from symptom onset to thrombolysis was similar in young versus older patients (6.1±2.4 hours vs 6.6±2.1 hours; p=0.36) (Table 1). The time from symptom onset to OCT was also comparable in young versus older patients (5.5±3.7 days vs 4.0±3.9 days; p=0.21).
Table 1. Baseline patient characteristics, angiographic findings, and treatment.
| ≤35 years (n=38) | >60 years (n=23) | p-value | |
|---|---|---|---|
| Baseline patient characteristics | |||
| Age, years | 29.9±4.2 | 64.5±4.2 | <0.0001 |
| Male | 36 (94.7) | 20 (86.9) | 0.29 |
| Current or former smoker | 24 (63.2) | 8 (34.8) | 0.03 |
| Diabetes mellitus | 3 (7.9) | 2 (8.7) | 0.91 |
| Hypertension | 2 (5.3) | 16 (69.6) | <0.0001 |
| Family history of coronary artery disease | 3 (7.9) | 0 (0) | 0.17 |
| Prior percutaneous coronary intervention | 0 (0) | 1 (4.3) | 0.20 |
| Symptom onset to thrombolysis, hours | 6.1±2.4 | 6.6±2.1 | 0.36 |
| Symptom onset to OCT, days | 5.5±3.7 | 4.0±3.9 | 0.21 |
| Angiographic findings | |||
| Culprit vessel | |||
| Left anterior descending artery | 29 (76.3) | 17 (73.9) | 0.83 |
| Diagonal branch | 2 (5.3) | 0 (0) | 0.27 |
| Left circumflex artery | 0 (0) | 4 (17.4) | 0.008 |
| Right coronary artery | 7 (18.4) | 2 (8.7) | 0.30 |
| Reference vessel diameter, mm | 2.44±0.86 | 2.13±0.62 | 0.15 |
| Minimal lumen diameter, mm | 1.52±0.65 | 1.07±0.28 | 0.007 |
| Diameter stenosis, % | 34.4±13.2 | 47.0±15.0 | 0.001 |
| Lesion length, mm | 26.2±11.5 | 32.0±11.5 | 0.06 |
| Treatment | |||
| Medical therapy | 17 (44.7) | 0 (0) | 0.0002 |
| PCI | 21 (55.3) | 23 (100) | <0.0001 |
| Stent length, mm | 32.8±13.3 | 32.8±9.5 | 1.0 |
| Stent diameter, mm | 3.3±0.5 | 3.0±0.4 | 0.01 |
| Medications at discharge | |||
| Aspirin | 38 (100) | 23 (100) | — |
| P2Y12 inhibitors | 36 (94.7) | 23 (100) | 0.26 |
| Statins | 38 (100) | 21 (91.3) | 0.06 |
| ACEi/ARBs | 35 (92.1) | 18 (78.2) | 0.12 |
| Beta blockers | 38 (100) | 15 (65.3) | 0.0001 |
| Values are mean±standard deviation or n (%). ACEi: angiotensin-converting enzyme inhibitors; ARB: angiotensin receptor blockers; OCT: optical coherence tomography; PCI: percutaneous coronary intervention | |||
BASELINE CHARACTERISTICS, ANGIOGRAPHIC FINDINGS, AND TREATMENT
Data are presented in Table 1. The mean age of patients in the ≤35 years age group was 29.9±4.2 years, while the mean age of the patients in the >60 years age group was 64.5±4.2 years. Most patients in the two groups were males (94.7% vs 86.9%; p=0.29). A history of smoking was more prevalent in very young patients compared with older patients (63.2% vs 34.8%; p=0.03), whereas hypertension was less common (5.3% vs 69.6%; p<0.0001) in very young patients. The left anterior descending artery was the most likely culprit vessel, followed by the right coronary artery. Angiographic stenosis was less severe and lesion length was shorter in very young patients compared with older patients.
OCT FINDINGS
Plaque erosion as the underlying mechanism of STEMI was more common in very young versus older patients (52.6% vs 21.7%; p=0.02) (Figure 2A). Plaque rupture was more common in older patients compared with very young patients (65.2% vs 36.8%; p=0.03). Calcified nodules were only present in 2 older patients. There were 5 patients whose culprit lesions were considered indeterminate; all of them had no thrombus or no late thrombus, and/or healed plaque within an entirely normal artery in 2 young patients, mild focal stenosis in 1 young patient, and diffuse stenosis in 1 older patient. When compared to very young patients, older patients showed a higher proportion of lipidic plaque (73.9% vs 42.1%; p=0.02) (Table 2). Fibrous plaques were more common in very young patients (52.6% vs 17.4%; p=0.008).
Acute or subacute thrombus was identified in 68.9% (42/61) of patients (Table 2). Red thrombus was more frequent in very young patients, but there were no statistically significant differences in the rates of acute or subacute thrombus. When we combined all patients and compared thrombus age between plaque rupture versus plaque erosion, acute thrombus was more frequent in plaque rupture compared with plaque erosion (62.0% vs 28.0%; p=0.01), whereas subacute thrombus was more common in plaque erosion versus plaque rupture (52.0% vs 10.3%; p=0.0008) (Figure 2B).
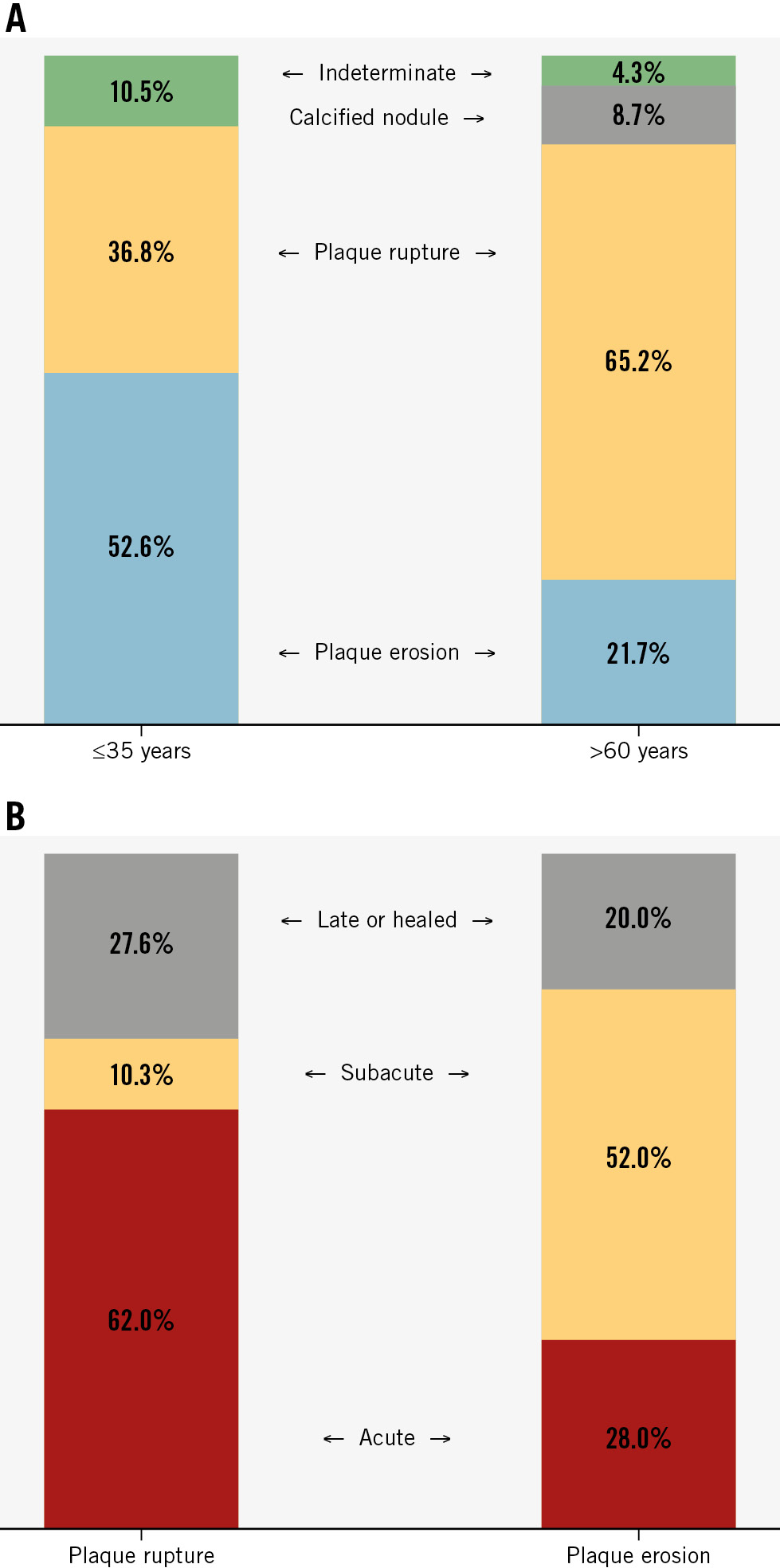
Figure 2. OCT features of the two groups. A) Underlying plaque types between very young versus older patients. B) Thrombus age between plaque rupture versus plaque erosion. OCT: optical coherence tomography
Table 2. Optical coherence tomography findings.
| ≤35 years (n=38) | >60 years (n=23) | p-value | |
|---|---|---|---|
| Minimum lumen area, mm2 | 1.9[1.3-3.8] | 0.9[0.8-1.2] | 0.0005 |
| Lumen area stenosis, % | 68.6 [57.0-85.0] | 75.0 [64.0-80.1] | 0.72 |
| Proximal reference lumen area, mm2 | 3.6[3.1-4.0] | 3.1[2.9-3.4] | 0.07 |
| Distal reference lumen area, mm2 | 2.9[2.5-3.3] | 2.5[2.2-2.7] | 0.001 |
| Lipidic plaque | 16 (42.1) | 17 (73.9) | 0.02 |
| Thin-cap fibroatheroma | 10 (26.3) | 9 (39.1) | 0.29 |
| Fibrous plaque | 20 (52.6) | 4 (17.4) | 0.008 |
| Calcified plaque | 0 (0) | 2 (8.7) | 0.14 |
| Normal artery | 2 (5.3) | 0 (0) | 0.52 |
| Any thrombus* | 28 (73.7) | 14 (60.9) | 0.29 |
| Thrombus type | |||
| Red thrombus | 8 (21.1) | 0 (0) | 0.01 |
| White thrombus | 20 (52.6) | 14 (60.9) | 0.53 |
| Thrombus age | |||
| Acute | 15 (39.5) | 11 (47.8) | 0.52 |
| Subacute | 13 (34.2) | 3 (13.0) | 0.08 |
| Late thrombus and/or healed plaque | 6 (15.8) | 8 (34.8) | 0.09 |
| Values are median [1st quartile-3rd quartile] or n (%). *Not including late thrombus and/or healed plaque. | |||
TREATMENT
All patients underwent thrombolysis within 24 hours from symptom onset. All older patients underwent percutaneous coronary intervention (PCI), whereas 55.3% of younger patients underwent PCI, and the rest were treated medically.
FOLLOW-UP
Six-month outcomes were confirmed for all 61 patients. There were no revascularisations in either group. One patient in the older age group was hospitalised because of heart failure with reduced ejection fraction at 28 days after STEMI. One patient in the very young age group died because of an out-of-hospital sudden cardiac arrest at 98 days after STEMI. Another patient in the older age group died due to an out-of-hospital sudden cardiac arrest at 124 days after STEMI.
Discussion
To the best of our knowledge, this is the first study to use OCT to show underlying culprit lesion pathophysiology in very young (≤35 years) adult STEMI patients. We report the following important findings. First, OCT imaging revealed that the mechanisms for STEMI in young and older adult patients were different. Plaque erosion was common in very young adult patients, while plaque rupture was seen in a greater proportion of older adult patients. Additionally, the typical findings of a vulnerable plaque were less likely to be observed in very young STEMI patients. Second, plaque rupture was associated with more acute thrombi, while plaque erosion had a higher frequency of subacute thrombi.
STEMI IN YOUNGER AND OLDER PATIENTS – INSIGHTS AND MECHANISMS
Yahagi et al15 showed in 236 sudden death autopsy cases with acute thrombi, plaque erosion was more prevalent in younger than older bodies (36.2% in <50 years vs 22.4% in ≥50 years). Among younger bodies, females suffered plaque erosion four times more often compared with males. Similarly, a large STEMI-OCT registry showed that the associated clinical factors for plaque erosion were younger age (<50 years) and current smoking16, which is consistent with our findings. Similar findings were reported by Fang and colleagues wherein younger STEMI patients (<50 years) were more likely to be current smokers with a greater frequency of dyslipidaemia (Supplementary Table 1)7. Smoking is a potent trigger for acute coronary thrombosis by altering endothelial function, platelet activation, and other homeostatic processes. It has been shown to be an important cause of plaque erosion and coronary thrombosis, especially in young men and premenopausal women17. In young patients with STEMI with minimal luminal narrowing, plaque erosion often leads to subclinical thrombosis, resulting in layered pattern plaques513. Four-year outcomes from the Effective Anti-Thrombotic Therapy Without Stenting: Intravscular Optical Coherence Tomography-Based Management in Plaque Erosion (EROSION) study showed that among 52 STEMI patients with plaque erosion and deferred stenting, 5 patients underwent target lesion revascularisation within 1 year, and an additional 6 patients were stented between 1 and 4 years after diagnosis1819. In young STEMI patients with plaque erosion and without significant luminal stenosis (residual diameter stenosis <70% on coronary angiography), effective antithrombotic therapy without stent implantation may be a definitive treatment option.
THROMBUS AGE ON OCT
In the majority of STEMI cases, occlusive luminal thrombosis is the predominant mechanism of ACS with >75% of patients with fatal ACS having thrombotic occlusion secondary to atherosclerotic plaque rupture1320. Systemic thrombolysis was used in our study as a part of the pharmacoinvasive procedure, as the majority of patients initially presented to a non-PCI centre. Earlier studies have highlighted the role of systemic thrombolysis in mortality reduction in STEMI21. Subjects with an occluded infarct-related artery having Thrombolysis in Myocardial Infarction (TIMI) grade 0 or 1 flow at 90 minutes post-thrombolysis were associated with an 8.9% 30-day mortality rate; subjects with TIMI grade 2 had a 7.4% mortality rate, and those with TIMI grade 3 flow (i.e., normal perfusion) had a 4.0% mortality rate. Previously, age of thrombus has been evaluated either on autopsy specimens13 or on the histopathology of the aspirated thrombus from the coronaries22. Kramer et al13, in their histopathological evaluation of coronary arteries in sudden cardiac death, reported that subjects with plaque rupture had a significantly greater degree of acute thrombus, while subacute and chronic thrombus was seen in those with plaque erosion, consistent with our study. The current study suggests that real-time identification of the age of thrombus based on OCT could potentially help in identifying the disease pathophysiology, determining treatment options, and improving outcomes.
CLINICAL IMPLICATIONS
Considering the distinct demographic characteristics and OCT pathologies of plaque rupture and plaque erosion in STEMI patients, individualised STEMI treatment strategies are of vital importance. Young STEMI patients with plaque erosion, <70% diameter stenosis, and TIMI 3 flow may be effectively managed with dual antiplatelet therapy alone as in the EROSION study. STEMI patients with plaque erosion on OCT often tend to have a better prognosis compared to those with plaque rupture23. Subjects with plaque erosion on OCT may be stabilised with medical therapy without stent implantation. This shift in the policy of management of STEMI patients with plaque erosion to antithrombotic therapies rather than PCI deserves consideration, especially in resource-limited countries such as India. This shall not only help in reducing procedure-related risks such as stent failure but also lead to a reduction in healthcare costs.
Limitations
The major limitation of the present study is the small sample size and single-centre design. Also, females were underrepresented in this study. OCT is hampered in the presence of large thrombus. The study patients were all from India; hence, extrapolation of the results of this study to various ethnic groups and larger populations is not possible. However, the South Asian population including Indians are known to suffer from ischaemic heart disease a decade earlier than other populations24.
Conclusions
Very young adult patients with STEMI treated with thrombolysis are characterised by a predominant fibrous plaque phenotype and erosion as the primary mechanism of STEMI with more subacute and chronic thrombus.
Impact on daily practice
Plaque erosion is the dominant underlying pathophysiology in very young patients with ST-segment elevation myocardial infarction. Patients with minimal residual stenosis and Thrombolysis in Myocardial Infarction 3 flow may be considered for medical therapy without immediate percutaneous coronary intervention.
Conflict of interest statement
A. Maehara reports consultant fees for Abbott, Boston Scientific, Philips, and SpectraWAVE; and speaker honoraria from Nipro. M. Matsumura reports consultant fees from Boston Scientific and Terumo. A. Qamar reports receiving institutional grant support from Novo Nordisk and NorthShore Auxiliary Research Scholar Fund; and fees for educational activities from the American College of Cardiology, Society for Vascular Medicine, Society for Cardiovascular Angiography and Interventions, Johnson & Johnson, Pfizer, Medscape, and Clinical Exercise Physiology Association. G.S. Mintz reports honoraria from Boston Scientific, Philips, Abbott, SpectraWAVE, and Gentuity. Z.A. Ali reports institutional grant support from Abbott, Abiomed, Acist Medical Systems, Amgen, Boston Scientific, CathWorks, Canon, Conavi, HeartFlow, Inari, Medtronic, National Institute of Health, Nipro, Opsens Medical, Medis, Philips, Shockwave Medical, Siemens, SpectraWAVE, and Teleflex Inc; consultant honoraria from Abiomed, AstraZeneca, Boston Scientific, CathWorks, Opsens Medical, Philips, and Shockwave Medical; and equity in Elucid, Lifelink, SpectraWAVE, Shockwave Medical, and VitalConnect. The other authors have no conflicts of interest to declare.

