A 71-year-old female with a history of congestive heart failure presented with acute onset of chest pain and New York Heart Association Class IV heart failure symptoms. A diagnosis of anterior ST-elevation myocardial infarction (STEMI) was made, and primary percutaneous coronary intervention (PCI) was arranged.
An echocardiogram showed a severely impaired left ventricular (LV) ejection fraction (EF) of 20-25%. A coronary angiogram showed an acute total occlusion in the proximal left anterior descending artery (LAD) with diffuse lesions over the left circumflex (LCx) and right coronary arteries (RCA). PCI was performed proximal to the LAD with a drug-eluting stent. Intraoperatively, the patient developed ventricular tachycardia requiring electrical cardioversion and cardiogenic shock (CS) requiring inotropic support. An intra-aortic balloon pump (IABP) was inserted, and poor peripheral vasculature was detected during its insertion (Figure 1A).
Her condition worsened postoperatively, evidenced by respiratory failure from acute pulmonary oedema, worsening of cardiogenic shock with rising lactate, oliguria, and acute liver injury with the increased use of inotropes. A repeat assessment showed a worsened LVEF of 10-15%, reduced cardiac power output of 0.5 W (≤0.6 W), and evidence of right ventricular (RV) failure (pulmonary artery pulsatility index 0.8). The decision was made to escalate mechanical circulatory support.
Several considerations were taken into account to decide on a suitable mechanical circulatory support approach for this patient with poor peripheral vasculature experiencing biventricular failure and respiratory failure. An upgrade to Impella (Abiomed) or biventricular Impella, a technique known as BiPella, would have had a financial impact and further limited vascular access, while the Impella CP alone would not have offered support to the RV. Conventional extracorporeal membrane oxygenation (ECMO) has drawbacks in terms of an increase in afterload due to the retrograde flow through the arterial cannula. The consequent rise in LV end-diastolic pressure and distension results in increased wall stress and oxygen demand, leading to impaired coronary perfusion and LV recovery. Another drawback is potential blood stasis in the LV, which can result in catastrophic thromboembolic complications and mortality. Therefore, an LV venting strategy is often needed when considering ECMO in cardiogenic shock, especially when prolonged support is anticipated; an IABP does not offer reliable venting, however, and Impella requires additional large-bore access, which was prevented by the poor vascular access in this case.
Left atrial (LA) venoarterial (LAVA)-ECMO was therefore chosen for this patient in order to solve these problems. The arterial cannula was inserted, replacing the IABP. Femoral venous access was obtained, followed by transseptal puncture under transoesophageal echocardiography guidance. A 0.035″ Amplatz extra-stiff wire was exchanged and directed to the left upper pulmonary vein (Figure 1B). Then, a multifenestrated 24 Fr venous cannula (VEFM024 [Edwards Lifesciences]) was inserted into the left atrium along the stiff wire. With the multiple side holes in the LA and right atrial (RA) sides, the venous cannula was therefore indirectly able to unload the LV (Figure 1B). Alternating oxygenated and deoxygenated blood was observed in the venous cannula at bedside, demonstrating that the cannula was correctly inserted in the LA and serving its venting function (Figure 1C, Moving image 1).
After LAVA-ECMO, there were haemodynamic improvements in cardiac power output, pulmonary artery pulsatility index, and wedge pressure, with normalisation of lactate on day 1. Subsequent staged PCI to the LCx and RCA was performed on day 5, and decannulation of LAVA-ECMO was performed on day 9.
The use of LAVA-ECMO provides biventricular support with the unique advantage of providing LV venting without additional large-bore access for unloading. This approach could reduce bleeding complications and sometimes overcome challenges in vascular access.
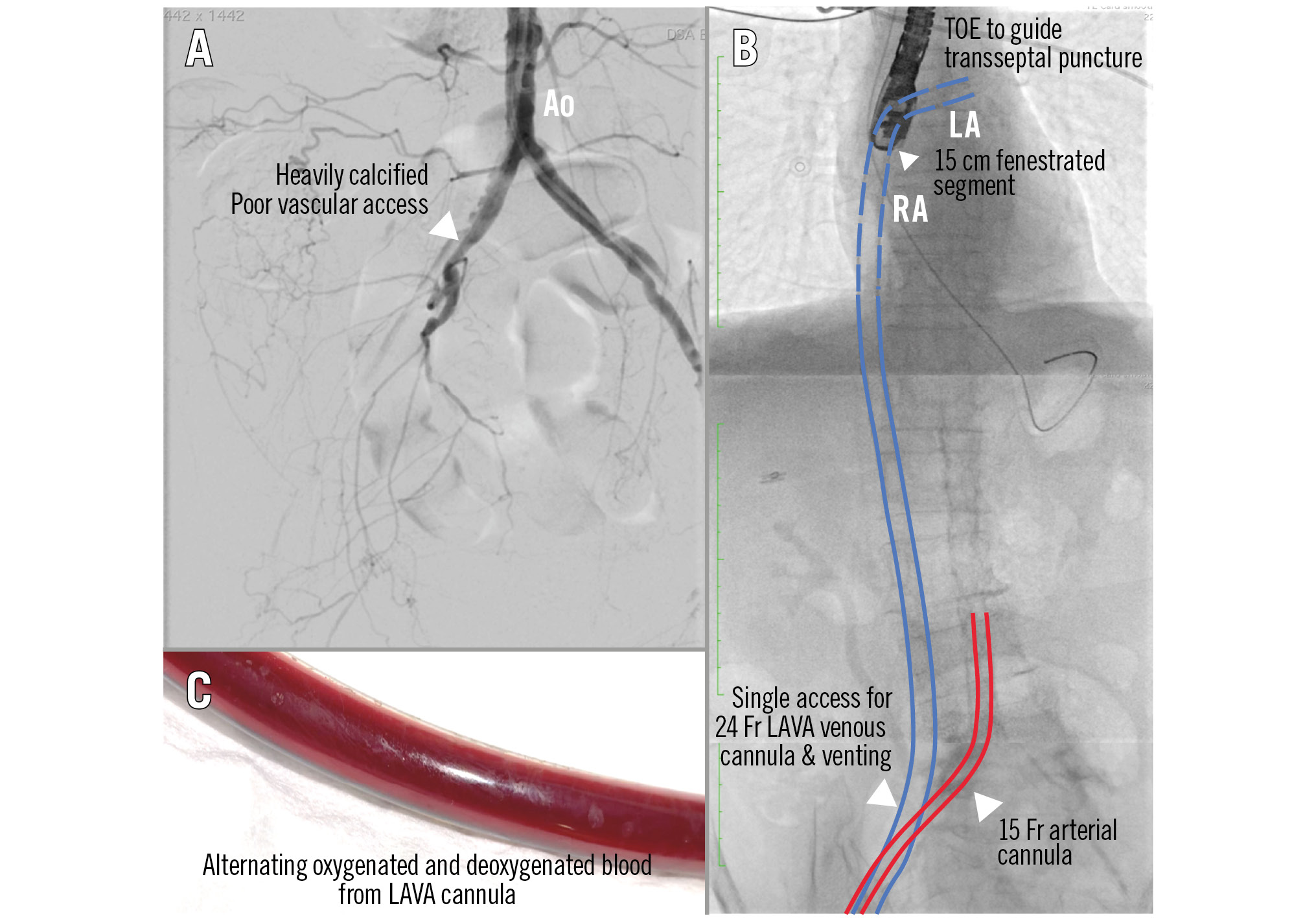
Figure 1. LAVA-ECMO provides biventricular support and LV venting in biventricular cardiogenic shock when there is poor vascular access. A) Poor peripheral vasculature. B) TOE-guided transseptal puncture, with an extra-stiff wire directed to the LUPV. Next, a 24 Fr multifenestrated LAVA cannula was inserted into the LA and a 15 Fr arterial cannula was exchanged for the IABP. C) Alternating oxygenated and deoxygenated blood was visible in the LAVA cannula. Ao: aorta; ECMO: extracorporeal membrane oxygenation; IABP: intra-aortic balloon pump; LA: left atrium; LAVA: left atrial venoarterial; LUPV: left upper pulmonary vein; LV: left ventricular; RA: right atrium; TOE: transoesophageal echocardiography
Conflict of interest statement
The authors have no conflicts of interest to declare.

