Introduction
An anomalous origin of the coronary artery is uncommon; it is encountered in 0.6–1.3% of catheterised patients12. There is a marked variation of the normal anatomic pattern in these patients. An anomalous right coronary artery (ARCA) arising from the opposite sinus has been reported in 8–27% of all patients with coronary anomalies12. Patients with ARCA may present in late adulthood with atherosclerotic obstructive coronary artery disease. The unusual location, take-off and intramural course of ARCA leads to considerable technical challenges during percutaneous coronary intervention (PCI). The selection of an appropriate guide catheter is critical to ensure selective angiography, assessment of obstructive lesions and adequate support during PCI. We hereby report our experience of PCI in 35 cases of ARCA from a tertiary care centre in Northern India.
Material and methods
We retrospectively analysed the PCI database over the last 14 years, from November 2007 to February 2022, and retrieved 35 PCI patients with ARCA. All the cases were performed by a team of interventionists under the supervision and active participation of the author R. Vijayvergiya. Details about demography, clinical presentation, PCI procedure and clinical follow-up were analysed. ARCA was suspected when the right coronary artery could not be selectively cannulated by Judkins, Tiger (Terumo) or Amplatz diagnostic catheters at the right coronary sinus. An aortic root angiogram or left coronary sinus injection in the left anterior oblique cranial view could help in localising the ARCA. Following localisation, a selective angiogram was attempted with diagnostic catheters to note the exact origin of the ARCA and the severity of coronary stenosis for PCI purposes. The ARCA was classified into the following 3 types, based on its relation to the left coronary artery (LCA) (Figure 1):
Type I: the ARCA origin was close to the LCA ostium. Both coronaries were simultaneously visualised on contrast injection.
Type II: the ARCA origin was distant from the LCA ostium, either near the midline separating the right and left coronary sinuses or from the ascending aorta above the left sinotubular plane.
Type III: the ARCA originated from the left main coronary artery. A single coronary ostium or single coronary trunk originated from the left coronary sinus, supplying the entire heart.
The study was approved by the institute’s ethics committee for retrospective analysis of the database. It conforms to the ethical guidelines of the Declaration of Helsinki.
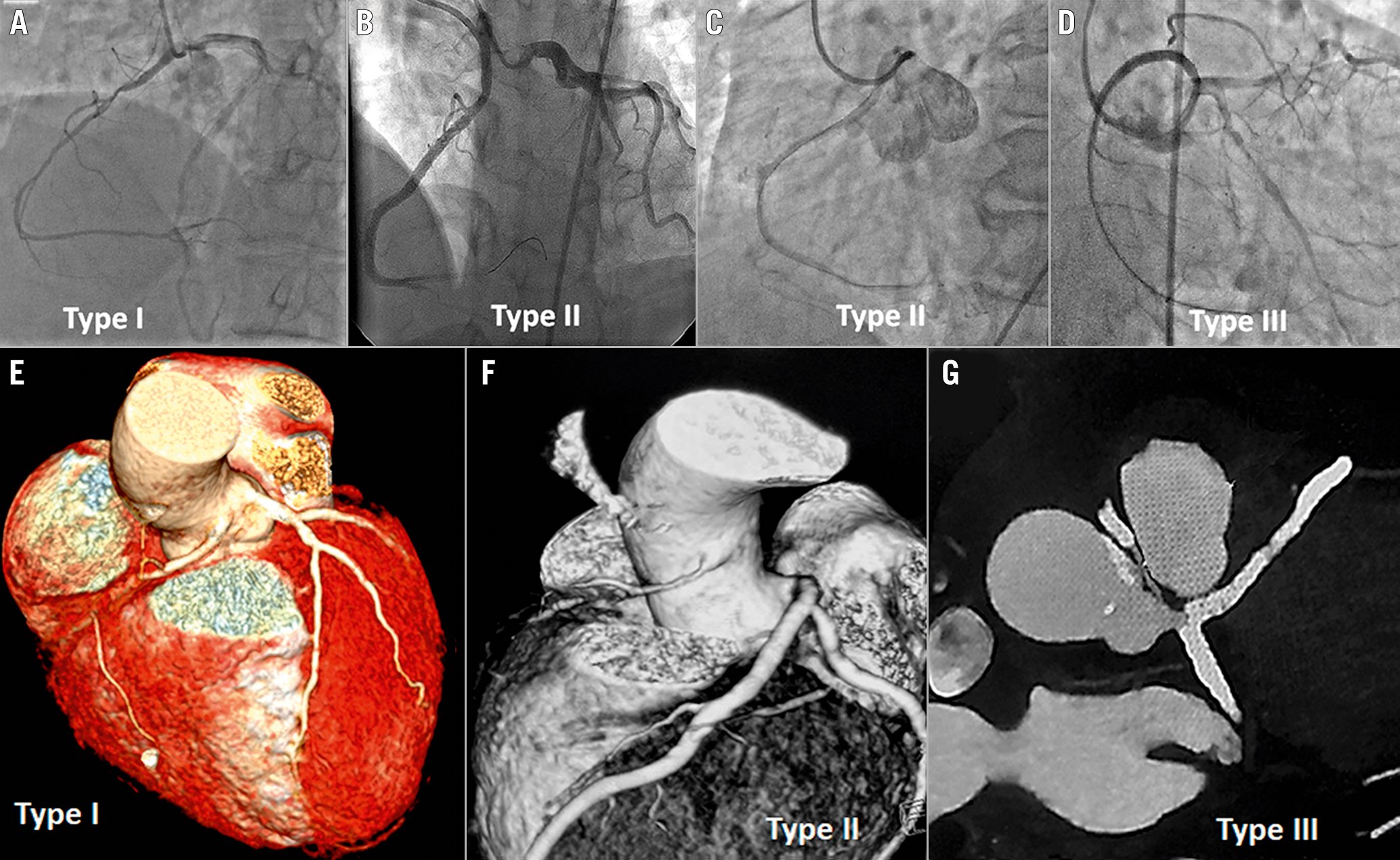
Figure 1. Various types of anomalous right coronary arteries. Coronary angiography (A-D) shows anomalous right coronary artery (ARCA) types arising from the left sinus of Valsalva. A) Type I ARCA arises near the ostium of the left coronary artery (LCA). B) Type II ARCA arises either from the midline or above the sinotubular junction (C) of the left sinus of Valsalva. D) Type III ARCA arises from the left main trunk. Computed tomography images show Type I (E), Type II (F) and Type III (G) ARCA arising from the left sinus of Valsalva.
Statistical analysis
Continuous variables were expressed as mean±1 standard deviation and median (interquartile range [IQR]) values. Categorical variables were expressed as percentages. The normality of the data was tested by the Shapiro-Wilk test. A Kaplan-Meier curve was constructed to analyse the cumulative survival and event rates.
Results
We found a total of 57 cases of ARCA out of 14,400 coronary catheterisations (0.39%) performed during the last 14 years. A total of 35 ARCA patients had PCI, which constitutes 0.53% of the total 6,600 PCI performed during the specified period. Out of 35 PCI patients, there were 25 males and 10 females, with a mean age of 56.7±13.5 years. Dyslipidaemia, hypertension and diabetes were the common risk factors, present in 54.3%, 45.7% and 34.3% of patients, respectively. The clinical presentation included stable angina in 45.7%, unstable angina in 20% and acute myocardial infarction in 34.3% of patients (Table 1). The most common ARCA was Type I, found in 51.4%, and the rest were Type II, in 42.9% and Type III, in 5.7% of cases (Table 2, Figure 1). Transradial and transfemoral access were used in 37.1% and 60.0% of cases, respectively. One patient in each group required crossover from transradial access to transfemoral access, or vice versa, as the ARCA could not be cannulated with the initial one. One patient (2.9%) had 7 Fr right transbrachial access. The characteristics of the coronary lesions were type C in 65.7% of cases, chronic total occlusion (CTO) in 14.3% and thrombus-containing lesions in 22.9% of cases (Table 2). The guide catheters used during PCI included the Judkins left (JL) (n=8; 44.4%) or Amplatz left (AL1) (n=9; 50.0%) for Type I (n=18); AL1 (n=12; 80%) for Type II (n=15); and JL (n=2; 100%) for Type III ARCA (Figure 2). All the guide catheters were size 6 Fr, except in 1 case of rotablation, where the AL1 7 Fr guide was used via right transbrachial access. Six patients required an exchange of 2 or more guide catheters before the selective cannulation. The diagnostic catheters used during coronary angiography also helped in the selection of the specific guide catheters used during the PCI. All had successful PCI except 1 case of mid-ARCA CTO, in which the guidewire could not be crossed. The mean stent diameter and mean stent length of the total 52 stents were 3.2±0.4 mm and 30.1±9.5 mm, respectively. Two patients with balloon-uncrossable, calcified CTO lesions had successful rotablation-assisted PCI. The technical details of the PCI of 1 rotablation case are demonstrated in Figure 3 and Moving image 1. Intravascular imaging was performed in 12 patients (34.3%), 8 (22.9%) had optical coherence tomography (OCT) and 4 (11.4%) had an intravascular ultrasound (IVUS) (Table 2). The intramural course, as evidenced by the proximal ARCA’s narrowing/elliptical shape in comparison to the mid-ARCA, was observed in 3 patients with OCT imaging (Figure 4). The slit-like, elliptical orifice was demonstrated in 4 patients with OCT imaging (Moving image 2). The rest of the patients with intravascular imaging had a normal circular ostium and an extramural course of the proximal ARCA (Figure 4). The mean radiation dose and fluoroscopy time were 877±687.3 mGy and 18.8±11.6 minutes, respectively. All patients had a satisfactory recovery following PCI and did not have any in-hospital clinical events. During follow-up, 6 patients expired and 1 was lost to follow-up. One patient died 5 days after discharge due to a hypoglycaemia-induced coma and aspiration pneumonitis. The remaining 5 patients died due to various non-cardiac causes including COVID-19 infection in 2, septic shock in 2 and chronic kidney disease in 1. The remaining 28 patients (80%) had an asymptomatic median follow-up of 49 (IQR: 29.0-97.5) months (Figure 5).
Table 1. Baseline characteristics of percutaneous coronary intervention patients with an anomalous right coronary artery.
| Baseline characteristics | N=35 | |
|---|---|---|
| Age (years) | 56.7±13.5* | |
| Male | 25 (71.4%) | |
| Coronary risk factors | Hypertension | 16 (45.7%) |
| Diabetes | 12 (34.3%) | |
| Smoking | 8 (22.9%) | |
| Family history of atherosclerotic CAD | 2 (5.7%) | |
| Renal dysfunction | 2 (5.7%) | |
| Chronic obstructive airways disease | 1 (2.98%) | |
| Peripheral arterial disease | 1 (2.9%) | |
| Dyslipidaemia | 19 (54.3%) | |
| Total cholesterol (mg/dL) | 154.1±42* | |
| HDL cholesterol (mg/dL) | 45.4±25.6* | |
| LDL cholesterol (mg/dL) | 94.1±35.6* | |
| Triglycerides (mg/dL) | 145.4±47.7* | |
| Clinical presentation | Acute myocardial infarction | 12 (34.3%) |
| Unstable angina | 7 (20.0%) | |
| Chronic stable angina | 16 (45.7%) | |
| Left ventricle ejection fraction by echocardiography (%) | 50.9±10* | |
| *Values are mean±1SD. CAD: coronary artery disease; HDL: high-density lipoprotein; LDL: low-density lipoprotein | ||
Table 2. Angiographic and procedural details for 35 patients with ARCA.
| Type of anomalous RCA | N=35 | |
|---|---|---|
| Type I | 18 (51.4%) | |
| Type II | 15 (42.9%) | |
| Type III | 2 (5.7%) | |
| Type of lesion | B1 lesion | 8 (22.9%) |
| B2 lesion | 4 (11.4%) | |
| C lesion | 23 (65.7%) | |
| Chronic total occlusion lesion | 5 (14.3%) | |
| Thrombotic lesion | 8 (22.9%) | |
| Procedural details | Right radial access (6 Fr) | 13 (37.1%) |
| Femoral access (6 Fr) | 21 (60.0%) | |
| Brachial access (7 Fr) | 1 (2.9%) | |
| Coronary guide catheter | Amplatz left 1 | 21 (60.0%) |
| Judkins left 3.5 | 9 (25.7%) | |
| Judkins left 4 | 2 (5.7%) | |
| Judkins left 4.5 | 1 (2.9%) | |
| Extra-backup left (EBU) 3.5 | 1 (2.9%) | |
| Judkins right 3.5 | 1 (2.9%) | |
| Change of guide catheter required | 6 (17.1%) | |
| No of stents per ARCA | 1.48±0.74 (total 52 stents) |
|
| Type of stents (n=52) | EES-44, SES-2, ZES-5, BMS-1 | |
| Mean stent diameter (mm) | 3.2±0.4* | |
| Mean stent length (mm) | 30.1±9.5* | |
| Rotablation | 2 (5.7%) | |
| Intravascular imaging | 12 (34.3%) | |
| Optical coherence tomography | 8 (22.9%) | |
| Intravascular ultrasound | 4 (11.4%) | |
| Additional PCI of other coronaries | 9 (25.7%) | |
| Fluoroscopy time (in min) | 18.8±11.6* | |
| Radiation dose (mGy) | 877±687.3* | |
| Follow-up duration in months (n=28) | 49 [29.0-97.5]# | |
| *Values are mean±1SD; #Values are median [interquartile range]. ARCA: anomalous right coronary artery; BMS: bare metal stent; EES: everolimus-eluting stent; PCI: percutaneous coronary intervention; RCA: right coronary artery; SES: sirolimus-eluting stent; ZES: zotarolimus-eluting stent | ||
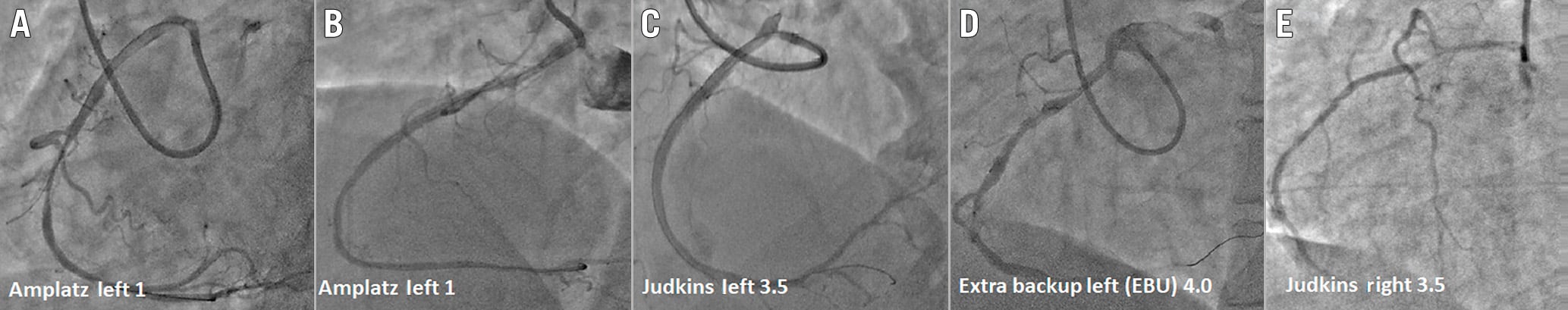
Figure 2. Various types of guide catheters used for selective cannulation of anomalous right coronary artery. Cannulation of anomalous right coronary artery with various guide catheters such as the Amplatz left (AL1) with (A) and without (B) aortic sinus support, with Judkins left (C), Extra-backup left (D) and Judkins right (E).
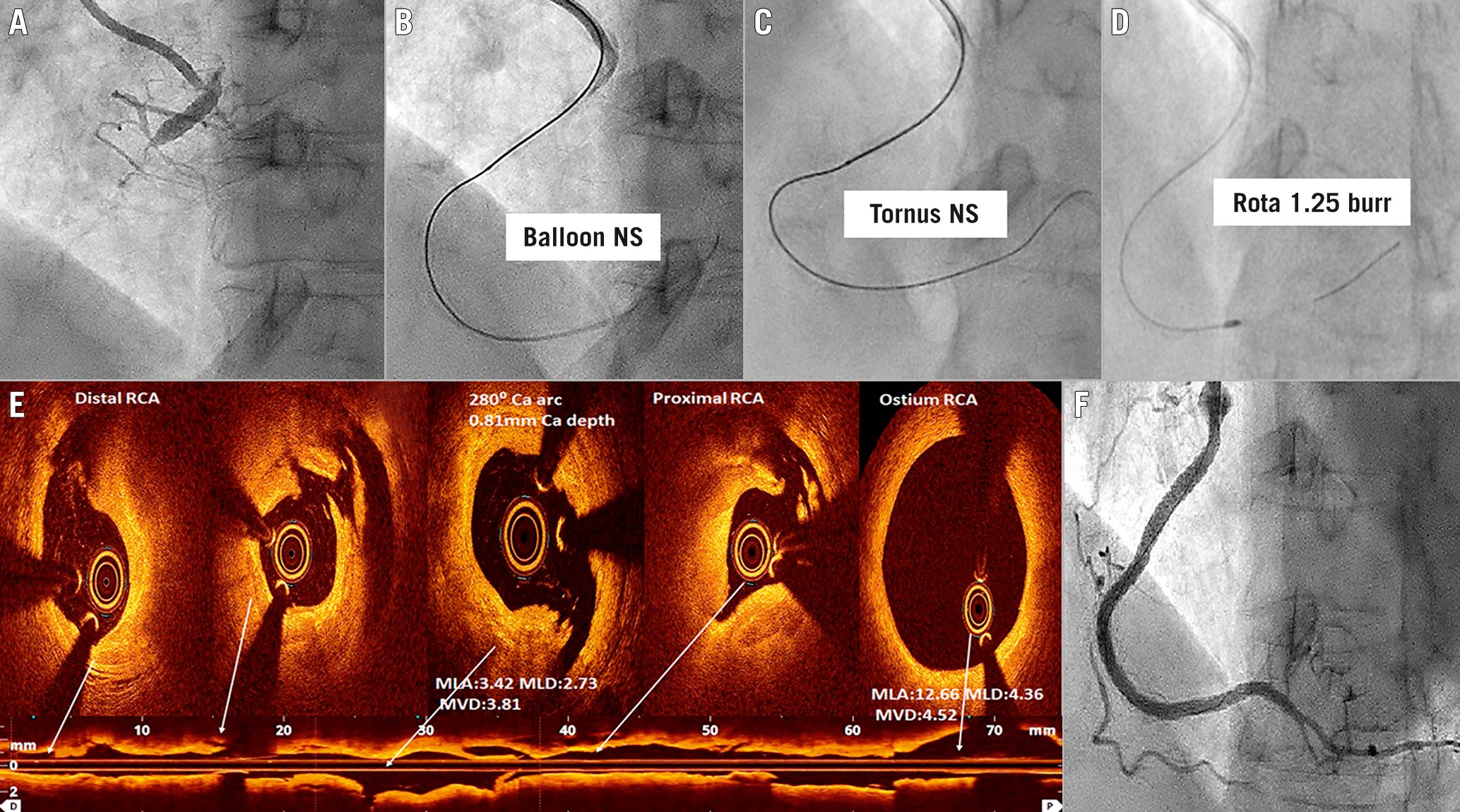
Figure 3. Percutaneous intervention of an anomalous right coronary artery chronic total occlusion lesion, using rotabalation and intravascular imaging. A percutaneous coronary intervention (PCI) case of anomalous right coronary artery (ARCA) Type II with chronic total occlusion (CTO) of the proximal part (A). ARCA was cannulated with AL1 6 Fr guide catheter via transradial access. The calcified CTO lesion could not be crossed with a small balloon (B) or Tornus microcatheter (Asahi) (C). Rotabalation with a 1.25 mm burr was successful (D). Optical coherence tomography (OCT) imaging of ARCA (E) showed fibrocalcific plaque with multiple iatrogenic dissections. The maximum calcium depth and calcium arc were 0.8 mm and 280°, respectively. The ostium of RCA was circular. There was no intramural course of proximal ARCA. Following drug-eluting stent implantation and OCT optimisation, there was a Thrombolysis in Myocardial Infarction (TIMI) 3 flow of ARCA (F). ARCA: anomalous right coronary artery; MLA: mean lumen area, mm2; MLD: mean lumen diameter, mm; MVD: mean vessel diameter, mm; NS: not successful; RCA: right coronary artery
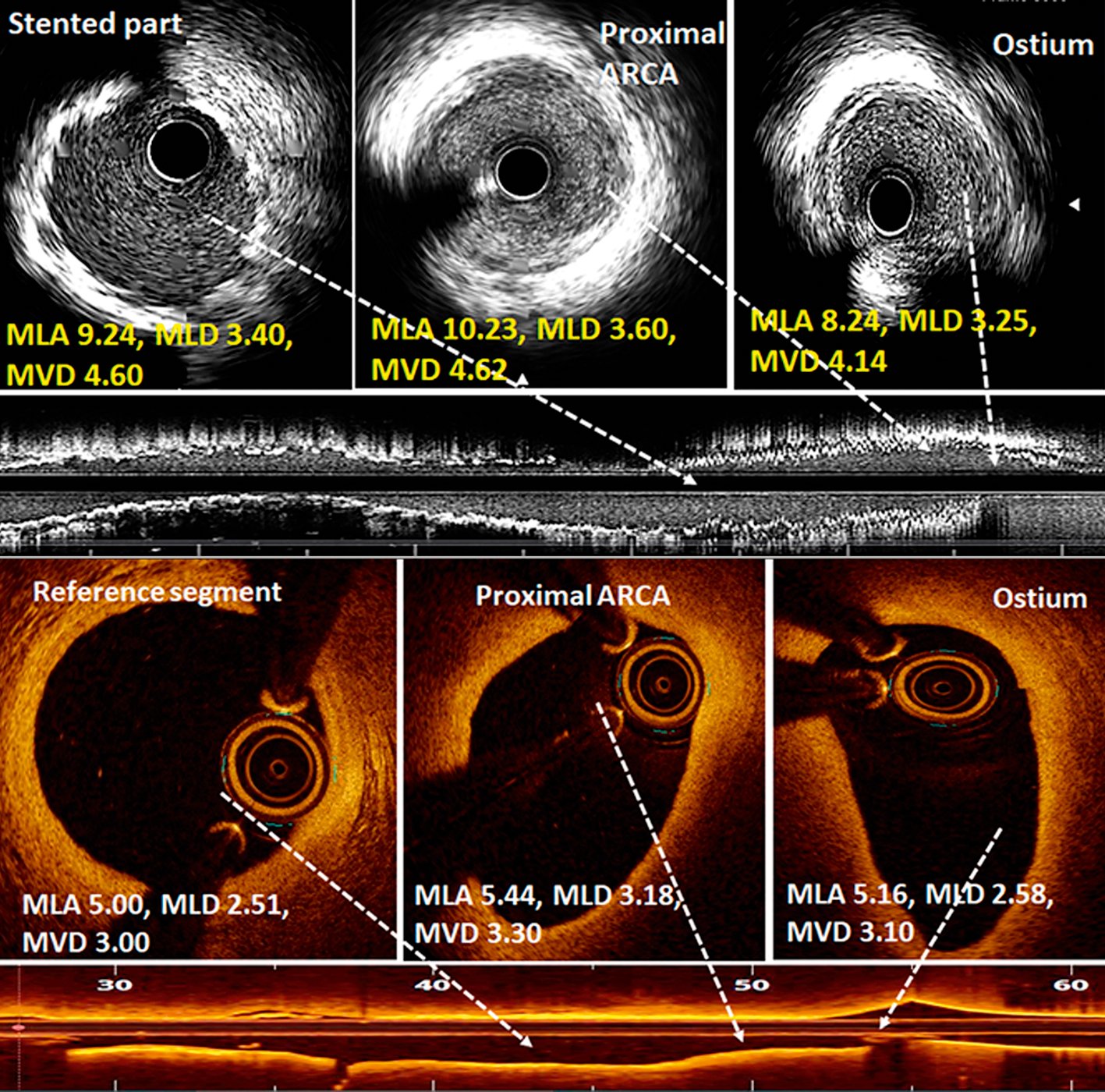
Figure 4. Intravascular imaging during PCI of anomalous right coronary artery. Post-PCI intravascular ultrasound images (upper panels) showed normal, circular ostium and proximal segment of an anomalous right coronary artery (ARCA). The mean luminal area (MLA) and mean luminal diameter (MLD) were comparable with the mid-stented part. Optical coherence tomography images (lower panels) showed the elliptical shape of the ostium and proximal part of ARCA in another case, suggestive of an intramural course. Though the proximal segment was elliptical, it was not stenosed as MLA and MLD were comparable with the mid-reference segment. MVD: mean vessel diameter, mm; PCI: percutaneous coronary intervention
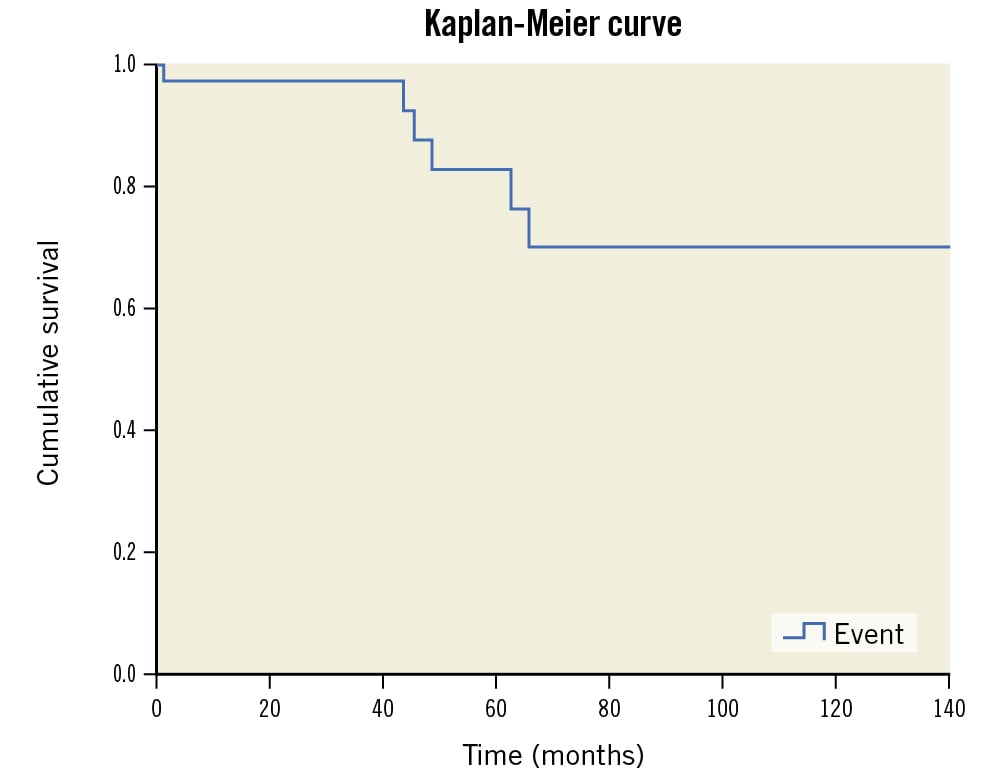
Figure 5. Kaplan-Meier curve showing the cumulative survival of 35 ARCA patients.
Discussion
ARCA patients can become symptomatic during adulthood due to atherosclerotic, obstructive disease. Its abnormal take-off and acute angle of origin make selective cannulation of ARCA a difficult task during PCI34. It is common for multiple guide catheters to be exchanged, to enable selective cannulation and adequate backup support during PCI. This increases the fluoroscopy time, radiation dose and contrast volume during the intervention34. A variety of standard and modified guide catheters have been used for the PCI of ARCA345. They include large and small curved JL, AL, Extra-backup left (Medtronic), and Voda Left (Boston Scientific) guide catheters. A manual modification of the AL1 and JL guide catheters’ tip with a 90-degree angulation for successful cannulation of ARCA has been reported by Angelini et al5 and Lin et al6. Active support from the aortic root and/or opposite aortic wall is required for selective cannulation and backup support in most cases. Sarkar et al divided ARCA into types A to D, depending upon the site of origin relative to the LCA ostium3. They also suggested particular guide catheters for individual types. We created 3 categories from I to III for better understanding and appropriate selection of guide catheters during PCI. Type I constitutes type B of Sarkar et al’s proposed classification3, while Type II constitutes types A, C and D3. Type III, the single coronary artery anomaly as added in the present study, was not described by Sarkar et al3. We found both JL and AL guide catheters suitable for Type I, AL for Type II, and JL for Type III ARCA. PCI of ARCA CTO is even more challenging as there is a lack of guide catheter backup support. Various techniques such as anchor balloon7, 7 Fr guide catheters3, “mother and child” catheters89, rotablation9, and even retrograde CTO intervention10 have been reported. We had successful PCI in 4 out of 5 CTO cases, 2 of which even required a rotablation for the balloon-uncrossable, calcified CTO lesion. Though transfemoral access is the preferred route for PCI34679, transradial access was also attempted by Lin et al6 and Yumoto et al8. We used right transradial access in 37% of patients. Among the 2 cases of rotablation, transradial 6 Fr access and transbrachial 7 Fr access were used in 1 case each. Intravascular imaging, specifically OCT, required selective cannulation to have a blood-free field, which was performed in 8 patients with good image acquisition. As demonstrated by Aubry et al11 and Häner et al12, we also demonstrated the slit-like ostial configuration and elliptical-shaped, proximal intramural course of ARCA by OCT imaging. Angelini et al5 and Driesen et al13 used IVUS and fractional flow reserve (FFR) to assess the significance of non-atherosclerotic narrowing of the ostium and the intramural course of ostial-proximal ARCA. We found a normal circular ostium and extramural course in 4 cases of IVUS imaging, including 2 cases of Type III ARCA. The mean fluoroscopy time of 18.8 minutes in the index study was lower in comparison to 20.7 minutes and 33.9 minutes as observed by Uthayakumaran et al4 and Sarkar et al3, respectively.
Limitations
Our study has certain limitations. It was a single-centre, retrospective, observational study. Though we were able to successfully perform the PCI with JL and AL guide catheters in most of the cases, and even with transradial access, other interventionists may not have similar technical success in such cases. An individual’s experience and technical skills play an important role for the technically challenging PCI of ARCA.
Conclusions
PCI of ARCA is technically challenging as it requires an appropriate guide catheter for cannulation and backup support during the percutaneous intervention. We performed successful PCI in a large cohort of 35 patients, including 2 patients with rotablation. PCI optimisation using intravascular imaging was also performed in 34.3% of patients. Though transfemoral access is commonly used for ARCA, 37% of our patients had transradial access. A favourable long-term clinical follow-up was observed in 80% of the cases.
Impact on daily practice
Though PCI of ARCA is technically challenging, appropriately chosen guide catheters can result in a successful intervention. Transradial access can be an alternative to the commonly used transfemoral access. PCI optimisation using intravascular imaging is also feasible following selective cannulation of ARCA.
Conflict of interest statement
The authors have no conflicts of interest to declare relevant to the content herein.
