Ventricular septal defects (VSDs) are among the most common congenital heart diseases, accounting for nearly one-third of all cases, with perimembranous VSD (pmVSD) being the most prevalent subtype1. Surgical closure of VSDs is widely performed but carries potential risks such as complete atrioventricular block, infection, and neurological issues2. Catheter-based interventions for muscular VSDs have shown promising results since 1988, with the youngest patient being an 8-month-old infant3, but data for perimembranous VSD closure are scarce.
This case report discusses the successful percutaneous closure of a VSD in a 2-month-old infant. To the best of our knowledge, it is the youngest age recorded for a successful transcatheter pmVSD device closure.
Percutaneous closure has been an effective treatment modality in paediatric patients, though it poses challenges in infants with low body weight or poor vascular access. This case report describes a successful percutaneous closure of a pmVSD using the Amplatzer Duct Occluder II (ADO II [Abbott]) device in a 2-month-old infant weighing 3 kg, underscoring the feasibility and safety of this approach in very low-weight infants.
A 2-month-old infant presented with breathing difficulty, tachycardia, and failure to thrive. Born via caesarean section at a birth weight of 2.65 kg, the infant weighed 3 kg at the time of presentation. Consanguinity was noted in the parents’ histories. The infant exhibited dyspnoea during feeding, irritability, and a grade 3/6 pansystolic murmur in the left parasternal area. A chest X-ray indicated mild cardiomegaly, with a left ventricular end-diastolic diameter of 18 mm, and echocardiography revealed a 5.5 mm perimembranous VSD with a peak gradient of 32 mmHg and a pulmonary artery systolic pressure of 35 mmHg. Despite medical therapy, the infant’s symptoms persisted, and due to failure to thrive, percutaneous closure was planned in consultation with a paediatric cardiovascular surgeon.
After informed consent, the patient was sedated and intubated. Preoperative aspirin (5 mg/kg) was administered the night before the intervention. Right femoral artery (RFA) and right femoral vein (RFV) access were obtained with a 4 Fr sheath. A 4 Fr pigtail catheter was then inserted on a polytetrafluoroethylene (PTFE) guidewire through the RFA to the ascending aorta and placed inside the left ventricle (LV), and an LV image was taken in the left anterior oblique (LAO) cranial view, which showed a VSD of size 5.2 mm; the pigtail was subsequently removed (Figure 1A). A 4 Fr Judkins right (JR) was inserted through the RFA over the guidewire and placed in the ascending aorta (Figure 1B). A Terumo wire was passed through the JR catheter, crossed the VSD, and progressed through the right ventricle (RV) to the right atrium (RA) and into the inferior vena cava (IVC). A Cocoon 6 Fr sheath (SMT) was inserted through the RFV, and a Terumo wire was snared inside the IVC with the help of an Amplatz Goose Neck snare catheter (Medtronic). The ADO II device, of size 6×4 mm, was passed through the delivery sheath across the VSD, and its LV disc was subsequently released under fluoroscopy (Figure 1C, Figure 1D). For confirmation of the proper device position, an LV image was taken (Figure 1E), followed by release of the device from the delivery cable (Figure 1F).
The patient tolerated the procedure well, though a blood transfusion was required due to blood loss from the access site. The patient was monitored for 24 hours in the intensive care unit before being discharged. Postprocedural echocardiography showed a well-seated device with no residual shunt, no pericardial effusion, and no conduction disturbances on electrocardiogram (ECG).
At the 4-week follow-up, the infant showed significant weight gain and improved feeding, with complete resolution of symptoms. Medications for congestive heart failure were discontinued at 6 weeks. Repeat echocardiography showed no residual shunt or valve regurgitation, and the ECG remained normal. The patient has been followed for two years with no complications.
Transcatheter closure of pmVSD has become a viable alternative to surgical repair in paediatric patients, particularly in older children. However, in neonates and small infants, the approach is more challenging technically because of issues with vascular access, device sizing, and proximity to critical structures such as the aortic valve and conduction system.
This case report highlights the successful use of the ADO II device in an infant weighing just 3 kg, making them one of the youngest and lightest patients to undergo this procedure.
Neonates and infants pose unique challenges due to their small size, requiring meticulous preparation, precise positioning and ultrasound-guided vascular access to reduce complications. A soft-tipped guidewire (e.g., 0.014 inch coronary wire) followed by the use of larger wires (0.018/0.021 or 0.025 inch) and cannulas can improve success rates. Whenever possible, a transvenous approach should be prioritised to minimise arterial complications. The use of a small, low-profile device like the ADO II, originally designed for patent ductus arteriosus closure, facilitated a safe and effective pmVSD closure in this case4.
Percutaneous closure of pmVSD is not without risks, including complete atrioventricular block (CAVB), aortic insufficiency, and tricuspid valve insufficiency. Device selection is critical, with the ADO II preferred for smaller defects (<6 mm LV entry) and the KONAR-MF device (LifeTech) for larger defects (>6 mm LV entry) and those with aneurysmal tissue. Both devices minimise CAVB risk, though continuous monitoring for late-onset conduction disturbances remains essential5.
Jiang et al observed that CAVB occurs in approximately 1% of cases, though rates have decreased over the years, and the most common postprocedural arrhythmia is incomplete right bundle branch block, which typically resolves without long-term complications6.
In our case, no device-related complications other than blood loss occurred. Other reports of transcatheter closure in infants as small as 3.2 kg corroborate the safety and efficacy of this approach7. Zartner et al reported an 88% success rate for transvascular VSD closure in infants <20 kg, though cases of acute CAVB and device malposition were noted8.
Transcatheter pmVSD closure using the ADO II device is a feasible and safe alternative to surgery in symptomatic infants with low body weight. The procedure, though technically demanding, can be performed successfully with appropriate device selection, careful vascular access, and vigilant monitoring for conduction abnormalities. Long-term outcomes are favourable, as evidenced in this case, with significant symptom resolution and no residual defects after two years of follow-up.
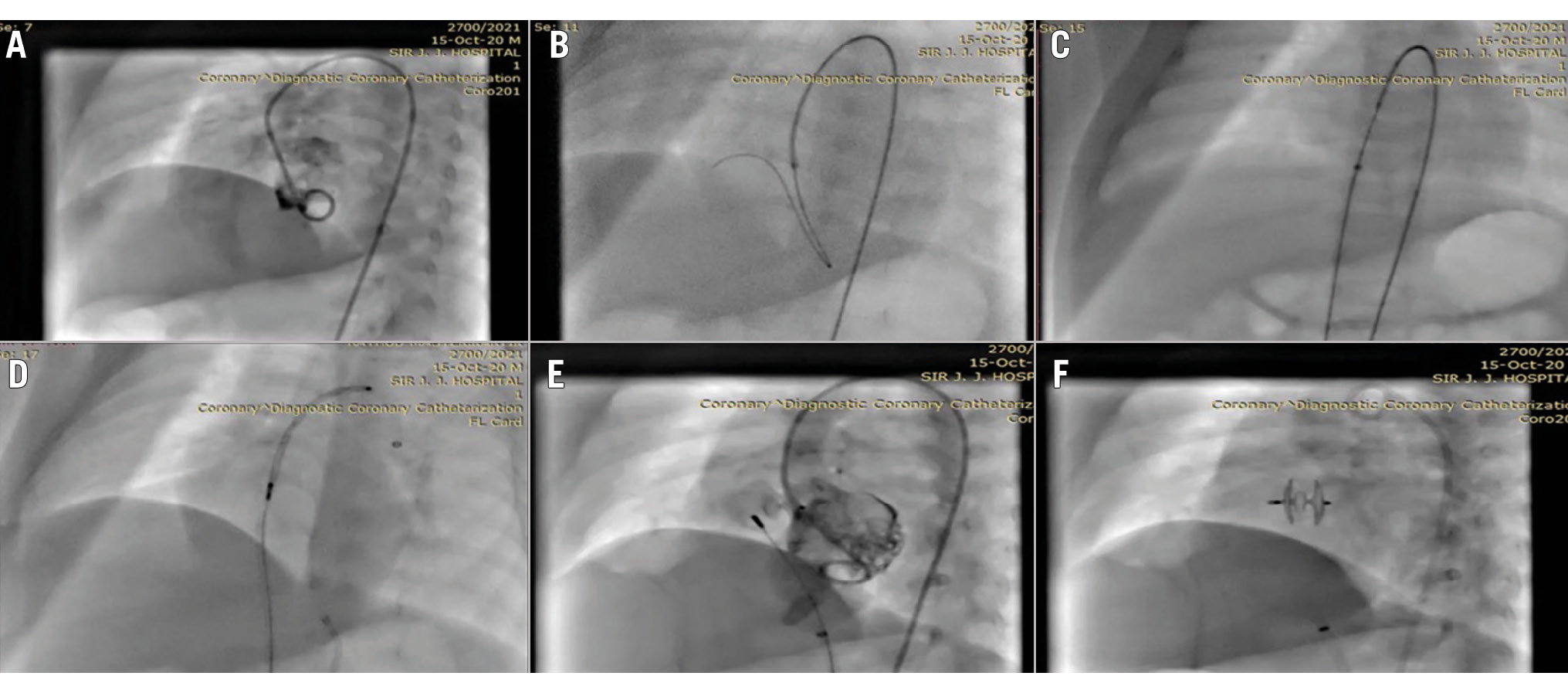
Figure 1. Cine fluoroscopic images of the steps of pmVSD device closure. A) LV view with pigtail catheter showing left-to-right shunt across the VSD. B) A Terumo wire attempting to cross the defect with a 4 Fr JR catheter. C) The Terumo wire snared across the defect and a Cocoon sheath (SMT) inserted for device delivery. D) ADO II device position across the defect. E) LV shoot showing no shunt across the VSD. F) Image showing device well placed across the defect. ADO II: Amplatzer Duct Occluder II; Fr: French; JR: Judkins Right; LV: left ventricular; pmVSD: perimembranous VSD; VSD: ventricular septal defect
Conflict of interest statement
The authors have no conflicts of interest to declare.

