Introduction
Percutaneous left atrial appendage closure (LAAC) has an established role as an effective alternative to oral anticoagulation (OAC) for the prevention of stroke and systemic thromboembolism in patients with non-valvular atrial fibrillation (NVAF). There has been exponential growth in the clinical acceptance of and indications for the procedure after its approval by the U.S. Food and Drug Administration in 2015. To date, more than 120,000 such interventions have already been performed worldwide, using various former and currently commercially available occlusive devices1.
Since 2012, the international guidelines2 indicate LAAC for the treatment of NVAF patients with high thromboembolic risk (as estimated by their CHA2DS2-VASc score) and contraindication to or at high risk for OAC, with a class IIb, level of evidence B recommendation. These same criteria and classes/levels of recommendation were maintained in the most recent international guidelines both for the treatment of patients with atrial fibrillation (AF)34 and for secondary prevention of stroke5. The II Brazilian Guidelines for Atrial Fibrillation are the only guidelines which, in addition to those same indications, also formally indicate LAAC as a therapeutic method for the prevention of stroke recurrence in patients who had a stroke of cardioembolic origin despite the correct use of OAC, with with a class IIa, level of evidence C recommendation6. Regarding patients with previous haemorrhagic stroke, the European guidelines recommend LAAC instead of resuming OAC for those patients who have irreversible causes or non-modifiable risk factors for intracranial bleeding, also with a class IIb, level of evidence B recommendation4.
There is no consensus on the definitions of absolute or relative contraindications to OAC. There are, however, some clinical scenarios where the majority of specialists agree that the use of OAC is associated with an excessively high risk of potentially fatal or disabling bleeding. Among those deserving mention are a history of previous significant bleeding or bleeding in noble organs (such as the spinal cord or intraocular); potentially non-correctable sources of significant bleeding in gastrointestinal (GI), pulmonary or urogenital tracts; coagulation disorders; chronic renal failure with a glomerular filtration rate (GFR) <15 ml/min; end-stage liver failure; presence of certain tumours; or the risk of frequent falls17.
Although the evidence derived from registries and randomised studies come from these classic indications, there are several other situations in which, despite the paucity of data, LAAC may be considered as a rational therapeutic strategy for the prevention of systemic thromboembolism in patients with NVAF (Table 1, Central illustration). Some of these will be discussed below.
Table 1. Indications for LAAC in patients with NVAF.
| Classic indications from the guidelines for the management of patients with NVAF and for secondary prevention of stroke | ||
|---|---|---|
| – Patients with high thromboembolic risk (as estimated by CHA2DS2-VASc score) and contraindication or high risk for OAC – Patients who had a stroke of cardioembolic origin despite correct use of OAC – Patients with previous haemorrhagic stroke and irreversible causes or non-modifiable risk factors for intracranial bleeding |
||
| Alternative indications in which LAAC may also be considered | Supporting reference | |
| Specific haemorrhagic diseases | Cerebral amyloid angiopathy | 1, 8, 10, 12, 13 |
| Age-related macular degeneration | none | |
| Hereditary haemorrhagic telangiectasia | 20-22 | |
| Moyamoya disease/syndrome | 26 | |
| LAA electrical isolation | 28-31 | |
| Persistent thrombus inside the LAA | 30, 34-38 | |
| End-stage renal disease | 39-45 | |
| Special groups of patients for whom lower rates of adherence to or persistence with OAC therapy may be anticipated | People living in areas with difficulty in accessing medical facilities | none |
| Homeless people | ||
| Patients who can’t afford medications | ||
| Patients with cognitive impairment and inadequate assistance | ||
| LAA: left atrial appendage; LAAC: left atrial appendage closure; NVAF: non-valvular atrial fibrillation; OAC: oral anticoagulation | ||
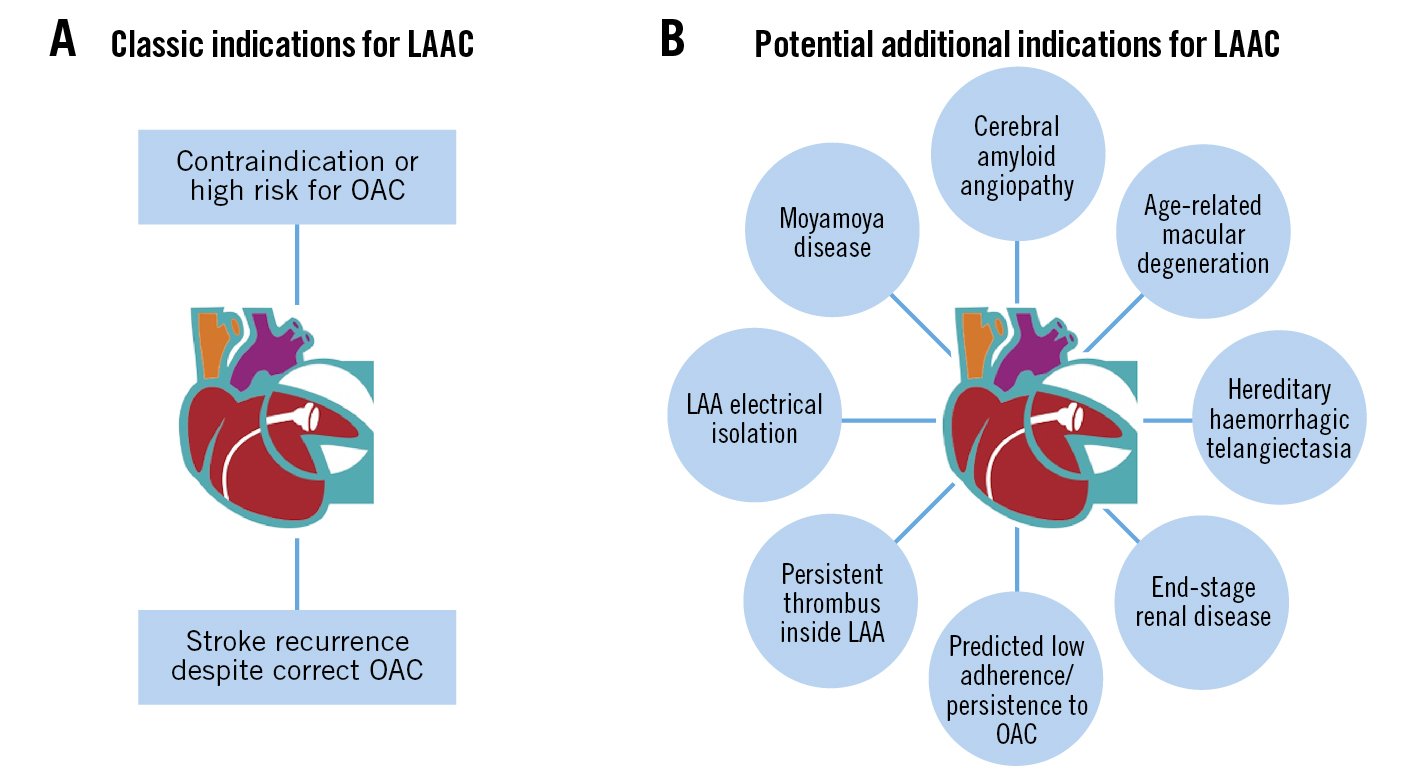
Central illustration. Left atrial appendage closure (LAAC); beyond the classic indications. Classic (A) and potential additional indications (B) for LAAC. LAA: left atrial appendage
Cerebral amyloid angiopathy
Cerebral amyloid angiopathy (CAA) is a haemorrhagic microvasculopathy which, together with hypertensive microangiopathy (Figure 1), is responsible for most cases of non-traumatic intracranial haemorrhages (ICH). Pathologically, the deposition of amyloid-beta 40 protein in the media and adventitia of cerebral vessels results in decreased vascular compliance and resistance, which in turn increases the susceptibility to cerebral microbleeds (CM). The prevalence of CAA increases with age, affecting 6.5% of people aged 45-50 and 36% of those over 80 years of age8.
Most patients with CAA will not experience significant events. However, although no cut-off number has been established, the presence of CM is associated with a 4-fold higher rate of symptomatic ICH9. Specific CAA phenotypes may also help to predict the chance of suffering ICH. These risk phenotypes are determined by the number of CM and the presence of cortical superficial siderosis, subarachnoid cortical haemorrhages and transient focal neurological episodes, and, depending on these combinations, they are associated with annual risks of symptomatic ICH that vary from 1 to 44%10.
The European guidelines for both the management of patients with AF and for the use of non-vitamin K antagonist oral anticoagulants (NOAC) in patients with AF acknowledge that the presence and number of CM are non-modifiable risk factors for the occurrence of ICH under OAC411. These guidelines, together with some expert opinions, recommend that patients with the so-called BLAST triad (CM, AF and stroke) should undergo a multidisciplinary evaluation by cardiologists and neurologists with regard to the risk-benefit ratio of maintaining OAC versus indicating LAAC181012. To date, there is only 1 publication in the literature on this specific topic: Schrag et al studied a cohort of 26 patients with AF and severe CAA (50% of them with a history of symptomatic ICH) who underwent LAAC and for whom no anticoagulation was prescribed beyond 6 weeks following the procedure. After the 25-month follow-up, there was no bleeding and only 1 ischaemic stroke, with complete recovery. The authors thus provided preliminary evidence that LAAC may be a safe and effective alternative for stroke prevention in this subgroup of patients13.
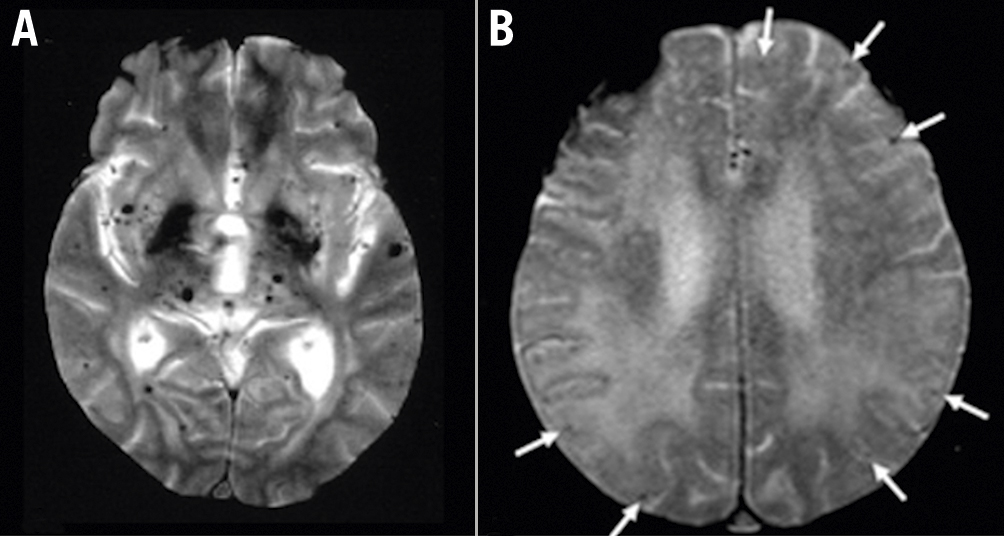
Figure 1. Cerebral amyloid angiopathy. Axial T2-weighted brain magnetic resonance imaging showing multiple black spots corresponding to perivascular haemosiderin deposits, compatible with microbleeds. A) Diffuse microbleeds, typical of hypertensive microangiopathy; B) cortical/subcortical microbleeds (arrows), typical of cerebral amyloid angiopathy. Images courtesy of Dr. VF Zétola, MD, PhD.
Age-related macular degeneration
Age-related macular degeneration (ARMD) is the leading cause of irreversible blindness in people older than 50 years in the Western world. This disease has a chronic course and presents in 2 forms: dry, atrophic or non-exudative; and wet, neovascular or exudative. Advanced wet ARMD is characterised by choroid neovascularisation with increased vascular fragility and permeability, leading to subretinal haemorrhages, fluid exudation and retinal detachment14 (Figure 2).
Deleterious consequences of OAC in patients with wet ARMD are controversial. Some studies demonstrate a strong association between antithrombotic therapy (both with antiplatelets or anticoagulants) and the development of large subretinal haemorrhages1516, while others do not prove this risk1718.
None of the above-mentioned guidelines for the management of atrial fibrillation and anticoagulants nor the guidelines for the management of neovascular ARMD by the European Society of Retina Specialists19 provide specific protocols of anticoagulation for this subgroup of patients. However, it seems prudent to suggest that patients with atrial fibrillation and severe or bilateral wet ARMD be anticoagulated with caution. For cases in which, after consultation with a cardiologist and an ophthalmologist, the net benefit of OAC is considered to be unfavourable, LAAC may be offered as an effective and less risky alternative for stroke prevention.
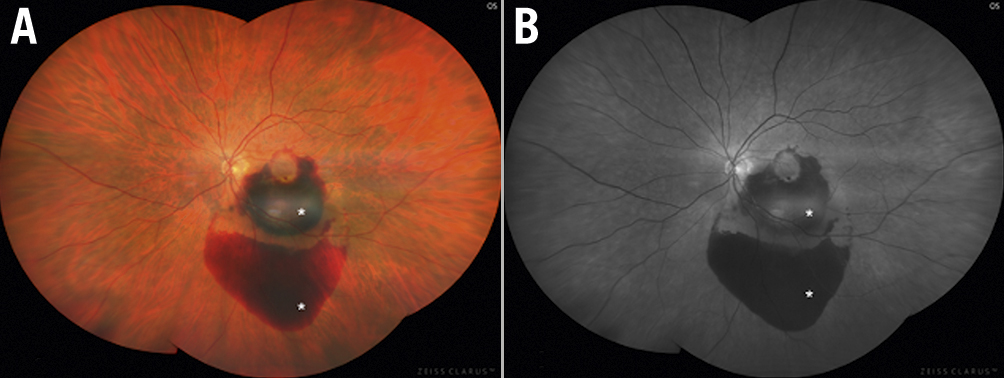
Figure 2. Age-related macular degeneration. Choroidal neovascularisation and subretinal haemorrhages (asterisks) associated with neovascular (wet) age-related macular degeneration, shown in (A) colour fundus photograph and (B) fluorescein angiogram.
Hereditary haemorrhagic telangiectasia
Hereditary haemorrhagic telangiectasia (HHT), also named Osler-Weber-Rendu syndrome, is an autosomal dominant genetic disease. Gene mutations result in vascular malformations ranging from small skin and mucosal telangiectases to large arteriovenous (AV) malformations in the liver, pancreas, lungs and brain that potentially lead to chronic bleedings, acute haemorrhages and clinical consequences of AV shunts. The most frequent HHT symptom is recurrent epistaxis, followed by gastrointestinal bleeding, haemoptysis, haemothorax and ICH2021.
Although the guidelines for the diagnosis and management of HHT state that this disease is not a formal contraindication for OAC in patients with NVAF (and that in this case vitamin K antagonists [VKA] are better tolerated than NOAC), the document suggests that these patients should be individually analysed with regard to their bleeding risk. In those patients for whom the net benefit of OAC is considered to be unfavourable, alternative approaches like LAAC should be adopted21. Two small case series reported good immediate- and medium-term results of LAAC performed in patients with HHT and NVAF, with effective protection both for bleeding and systemic thromboembolism2022.
Moyamoya disease
Moyamoya disease (MMD) is a genetic disease, the inheritance of which is autosomal dominant with incomplete penetrance. It is more prevalent in countries in Eastern Asia, such as Japan and Korea, but it also affects non-Asian people. The word “moyamoya” derives from a Japanese expression that means something hazy and indistinct, like cigarette smoke drifting in the air. MMD is characterised by bilateral stenosis of the supraclinoid portion of the internal carotid artery, which causes the development of a compensatory vascular net of collaterals (moyamoya vessels) at the base of the brain. Though a causal relationship has not been formally proven, similar vascular patterns can also be observed in association with neurofibromatosis, Down syndrome, sickle cell disease and after exposure to radiation – these patients are said to have Moyamoya syndrome. If no associated condition is found, the patients are said to have Moyamoya disease523.
There are 2 types of clinical manifestations related to the vascular pattern of the disease: ischaemic and haemorrhagic. Children most often have ischaemic symptoms. Half of adult patients present with ischaemia and the other half with cerebral bleeding. However, ICH predominates in patients over the age of 40. The prognosis of haemorrhagic MMD is mainly related to the recurrence of bleeding episodes, which occur at yearly rates of 7%24. The use of extracranial-intracranial bypass (used in the treatment of ischaemic MMD) for the prevention of rebleeding in these patients showed only marginal benefits25.
There are no specific recommendations about OAC for patients with MMD and NVAF, and there is only 1 reported case of LAAC performed for a patient with MMD, NVAF and recurrent stroke26. However, extrapolation of the considerations made above for patients with CAA, ARMD and HHT may suggest a potential role for LAAC in the management of patients with haemorrhagic MMD and NVAF.
Left atrial appendage electrical isolation
Several studies have shown that left atrial appendage electrical isolation (LAAEI) associated with pulmonary vein isolation in patients with permanent AF is beneficial in achieving freedom from all atrial arrhythmias at follow-up2728. However, in most cases LAAEI compromises the mechanical function of the left atrial appendage (LAA), leading to blood stasis and thrombus formation. Thrombus in the LAA may be found in more than 20% of patients who undergo LAAEI, despite maintenance of sinus rhythm29. Although OAC seems to be effective in preventing thromboembolism in this scenario, there is a 10-fold increase in the stroke risk of these patients if OAC regimens are not adequate, irrespective of their CHA2DS2-VASc score28.
LAAC proved to be effective for stroke prevention in patients undergoing LAAEI, both as an alternative to OAC for patients with high bleeding risk, as well as for the treatment of persistent thrombi in the LAA despite adequate OAC28293031. The procedure may be done at the same time as ablation, in an attempt to diminish the risks associated with a new intervention and to avoid the prolongation of OAC. Some studies, however, recommend LAAC to be performed 4-6 weeks after LAAEI. This strategy allows time for re-endothelialisation and oedema regression and also permits re-isolation of the LAA in case of recovered conduction, which may happen in as many as 37% of patients293132.
Persistent thrombus inside the LAA
The presence of thrombus inside the LAA despite optimal OAC is a relatively frequent finding. A meta-analysis of 35 studies, encompassing more than 15,000 patients with AF who underwent transoesophageal echocardiography after at least 3 weeks of adequate OAC, showed a prevalence of LA thrombi of 2.73%, no matter whether OAC was performed with VKA or NOAC. This prevalence was higher in patients with non-paroxysmal AF and in those whose CHA2DS2-VASc score was ≥333.
Thrombi inside the LAA were originally considered a contraindication to LAAC, and treatment was restricted to the intensification of antithrombotic therapy. However, this strategy results in thrombus resolution in only 75% of cases, and it is associated with increased bleeding risk and stroke rates when compared to off-label LAAC, despite the presence of local thrombus34.
Although still performed on an exceptional basis, and given the possibility of a reporting bias, recent publications demonstrate the feasibility, efficacy and safety of LAAC in the presence of persistent LAA thrombus, especially when using the “no touch” technique, in which the manipulation of the LAA is restricted to the minimum. Most of the patients had distally located thrombi, although some LAA with proximal thrombi were also treated. The implanted devices were mostly those intended for proximal deployment in the LAA (Amplatzer Cardiac Plug/Amplatzer Amulet [Abbott], LAmbre [LifeTech Scientific] and WATCHMAN FLX [Boston Scientific]), as this method of implantation is more compatible with the “no touch” technique. However, the earlier version, WATCHMAN 2.5 (Boston Scientific), was also used with good results, despite the need for deep implantation inside the LAA. These series demonstrated success rates of 98-100%, with no cases of intraprocedural thromboembolism, despite the use of cerebral protection devices in only 9-21% of cases3034353637. However, 1 study specifically investigated the role of cerebral protection devices in these procedures and found macroscopically visible debris in 29% of cases38.
In summary, instead of contraindication, the presence of persistent thrombus inside the LAA may turn out to be an additional indication for LAAC, provided the other possible management steps and alternatives (e.g., checking for compliance, intensification or switching of the OAC regimen) are also taken into consideration. The procedure has been demonstrated to be safe and effective, provided specific implantation techniques are employed (Figure 3). Cerebral protection devices probably play an important adjuvant role in the safety of this particular intervention.
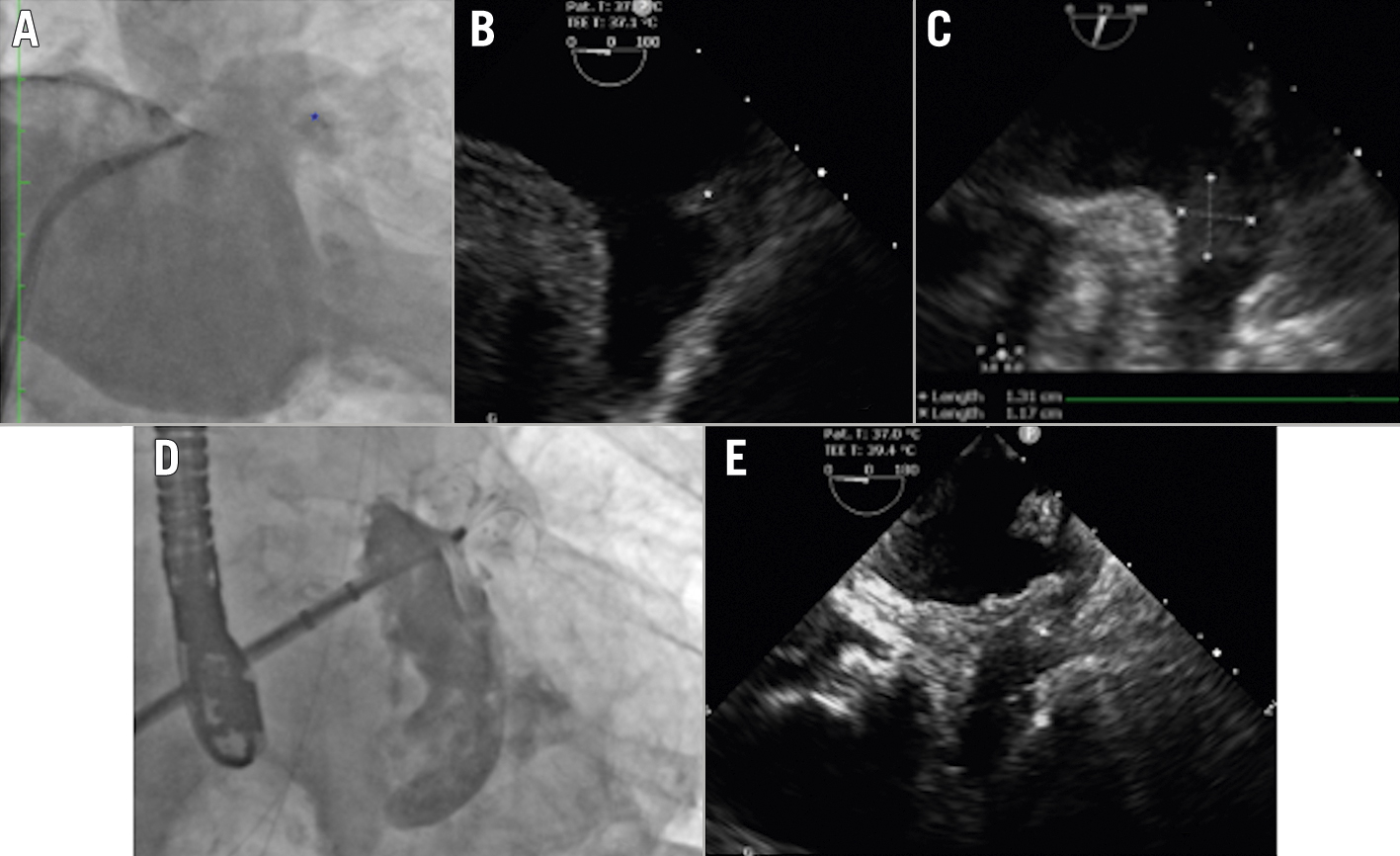
Figure 3. Persistent thrombus inside the left atrial appendage. Non-selective LAA angiography (A) and intraprocedural TOE (B,C) showing proximal LAA thrombus (asterisks). D) Angiographic result after LAA closure with the LAmbre device using the no-touch technique. E) Intraprocedural TOE showing the thrombus entrapped by the closure device (asterisk). Echo images courtesy of Dr. EM Balbi, MD. LAA: left atrial appendage; TOE: transoesophageal echocardiography
End-stage renal disease
The prevalence of AF in patients with end-stage renal disease (ESRD) is higher than in patients with normal renal function, ranging from 13 to 27%. However, AF-ESRD patients are a special group of patients who have simultaneous indications for and contraindications to OAC therapy. Due to higher levels of fibrinogen and other prothrombotic factors, ESRD patients have an increased risk of thromboembolic events. On the other hand, they also carry higher risks of bleeding, due to altered platelet function, uraemia and enhanced endothelial production of nitric oxide39.
Data about the safety and effectiveness of OAC in patients on haemodialysis are conflicting and scarce, leading to (if ever) a conservative prescription and frequent discontinuation of the therapy. Current guideline recommendations regarding anticoagulation in patients with AF and an estimated GFR (eGFR) <30 ml/min are inconclusive34, and the net clinical benefit of OAC therapy in these patients has never been evaluated in a randomised trial. Despite having a mainly hepatic metabolism, VKA failed to demonstrate the benefits of stroke prevention in ESRD patients: the time percentage with an international normalised ratio (INR) within the target range is lower than in patients with normal renal function. Additionally, VKA use in ESRD patients may indeed increase bleeding and stroke rates, as well as result in calcification and occlusion of cutaneous arteries4041. Reduced dose regimens of NOAC (which are in part eliminated by the kidneys) are feasible OAC options for patients with severe chronic kidney disease (creatinine clearance [CrCl]=15-29 mL/min). However, patients with ESRD (CrCl <15 mL/min and/or dialysis) were excluded from the pivotal NOAC trials and the scarce available data preclude a definitive answer regarding the safety and efficacy of NOAC in this clinical situation11. The results of the ongoing AXADIA trial (ClinicalTrials.gov: NCT02933697), which compares the results of apixaban versus VKA in NVAF patients on haemodialysis, may shed some light on this issue.
Few studies have specifically addressed LAAC in ESRD patients. However, procedural results are encouraging, with no differences in terms of success, residual leaks or complication rates in comparison with patients with normal renal function. Also, the efficacy of LAAC on the prevention of thromboembolic and bleeding events at follow-up seems to be as high in ESRD patients as it is in patients with normal renal function39404142434445. Luani et al42 found a trend towards higher rates of device-related thrombosis (but with no episodes of embolisation) in patients with a GFR <30 mL/min/1.73m2, and the German LAARGE registry45 demonstrated a lower survival rate free of stroke in chronic kidney disease patients (with no accentuation in ESRD patients), but these findings were not confirmed in other large multicentre studies404344. Taken as a whole, these results reinforce the potential role of LAAC as an alternative therapy to OAC in this high-risk group of patients who, for the most part, would in fact not be anticoagulated.
Adherence/persistence issues in special groups of patients
Adherence to (or compliance with) a specific medication is measured as the percentage of doses of medication taken in accordance with the prescription. In other words, it refers to the daily observation of timing, dosing and frequency of drug administrations. Persistence, in turn, refers to the continuation of the treatment over the prescribed length of time. In other words, it refers to the time elapsed between the beginning and discontinuation of the therapy46.
Rates of both adherence to and persistence with OAC in patients with AF are much lower than expected, which translates into higher stroke and mortality rates. Banerjee et al studied almost 37,000 NVAF patients from the UK Primary Care electronic records and determined 1-year rates of adherence to and persistence with OAC of only 55.2% and 65.9%, respectively47. Regarding adherence, Yao et al analysed a large US commercial insurance database encompassing more than 65,000 NVAF patients for whom OAC was prescribed. After 1 year of follow-up, only 47.5% of the NOAC patients and 40.5% of the warfarin patients adhered to the medication ≥80% of the year. Lack of adherence to medication was associated with up to a 4-fold higher risk of stroke, depending on individual CHA2DS2-VASc scores and compliance profiles48. Regarding persistence, Toorop et al investigated more than 93,000 NVAF patients from the Dutch national statistics. The authors showed that persistence with OAC decreased from 88.1% at 1 year to 72% after 4 years. In this population, non-persistence was associated with a 58% increased risk of ischaemic stroke and a 79% increased composite risk of ischaemic stroke and ischaemic stroke-related death49.
If even in the general NVAF population in developed countries OAC adherence and persistence rates are far less than ideal, there are specific groups of patients for whom this problem is certainly more worrisome, such as people living in rural areas with difficult access to medical facilities, homeless people, patients who cannot afford medications, or patients with cognitive impairment and inadequate assistance50. LAAC has been nominated as a “mechanical vaccine” against stroke and ICH because it precludes compliance to an OAC regimen51. For this reason, NVAF patients for whom low adherence and persistence profiles are anticipated might theoretically benefit from LAAC as a primary alternative to OAC, irrespective of the presence of some intolerance to the medication.
Conclusions
Besides the classic LAAC indications established in the guidelines, there are many other clinical situations in which this procedure might be considered appropriate, whether because of benefits already demonstrated by clinical experience, or potential benefits that can be anticipated through extrapolation of good results obtained in other scenarios. It should be emphasised, however, that most of these proposed alternative indications are based on a very low number of patients without long-term data. Accordingly, specific studies on new indications for LAAC could provide an alternative for the management of many patients who otherwise remain at constant risk of suffering a stroke.
Conflict of interest statement
E.E. Guérios received consulting fees/honoraria from Abbott Vascular, Lifetech Scientific, and Boston Scientific; and is proctor for left atrial appendage closure for Abbott and Lifetech Scientific. F. Chamié received consulting fees from Abbott Vascular and Lifetech Scientifc; honoraria from Lifetech Scientific; and is proctor for left atrial appendage closure for Abbott and Lifetech Scientific.

