Introduction
Since its first use, transcatheter aortic valve implantation (TAVI) has become an established treatment for symptomatic aortic stenosis (AS) with broad clinical indications123456. Along with the change in candidates for TAVI from high- to low-risk patients, current clinical practices should take into consideration the long-term management of TAVI patients. Following from this, valve durability has become an issue of concern, particularly in patients with a longer life expectancy. Recently, the Valve Academic Research Consortium (VARC)-3 proposed an updated endpoint definition of aortic valve clinical research including bioprosthetic valve dysfunction (BVD) and bioprosthetic valve failure (BVF)7. In the previous data, supra-annular prostheses demonstrated an excellent haemodynamic performance as compared to intra-annular prostheses in surgical and transcatheter aortic valve replacement89. Moreover, the data from the UK-TAVI registry demonstrated that TAVI with predilatation was associated with a decrease in valve dysfunction, although it was not confirmed after multiple tests in the short-term follow-up data10. Although it has not been thoroughly studied so far, valve design and/or certain procedural steps during TAVI may have an impact on valve durability. Therefore, we sought to evaluate whether the valve design and procedural steps of TAVI may have an impact on BVD/BVF.
Methods
Study population
From January 2017 to December 2020, a total of 530 patients underwent TAVI with balloon-expandable and self-expanding valves at Teikyo University Hospital in Tokyo, Japan. TAVI was performed via transfemoral or an alternative access based on preprocedural computed tomographic images and other imaging modalities. Predilatation and post-dilatation were not mandatory and were performed at the operator’s discretion. An echocardiographic assessment was mandatory at baseline and predischarge. At 1-year post-procedure, the echocardiographic assessment was left to the discretion of the doctors who performed the follow-up at the outpatient clinic. Survival status was checked through medical records or telephone calls. Patients who did not undergo post-procedural echocardiography, who were enrolled in a preclinical trial of a premarket transcatheter heart valve, or who experienced in-hospital death were excluded from this analysis. The study protocol was developed in accordance with the Declaration of Helsinki and was approved by the ethics committee of our hospital (TEIRIN 14-196). All patients gave informed consent before participating in this study.
TAVI device and procedure
Selection of a TAVI device, including a balloon-expandable valve (SAPIEN 3; Edwards Lifesciences) or a self-expanding valve (Evolut PRO; Medtronic), was done using multidetector computed tomography (MDCT) images of the native aortic valve anatomy. Device size was determined largely based on the annular measurements from MDCT images. If the use of a contrast medium could not be tolerated due to advanced chronic kidney disease, the annular measurement was performed using transoesophageal echocardiography. The annular and left ventricular outflow tract sizes were measured based on the previous study11. The choice of predilatation and/or post-dilatation was left to the operator. The operators took the following situations into account when deciding whether to perform predilatation: 1) severe calcification, 2) bicuspid valve, 3) very severe aortic stenosis, and 4) haemodynamically instable cases. To avoid annulus rupture, the size of the predilatation balloon did not exceed the short-axis diameter of the annulus, as measured by MDCT or transoesophageal echocardiography, per our practice.
Study endpoints and definitions
The primary objective of this analysis was to investigate the rates of BVD, haemodynamic valve deterioration (HVD), and BVF according to the VARC-3 criteria7. In brief, the types of BVD were categorised as follows: (a) structural valve deterioration (SVD), indicating an intrinsic permanent change of the prosthetic valve; (b) non-SVD, which includes a non-intrinsic abnormality of the prosthetic valve resulting in valve dysfunction (e.g., prosthesis-patient mismatch or paravalvular regurgitation); (c) clinically significant thrombosis; and (d) endocarditis. Prosthesis-patient mismatch and paravalvular regurgitation were also defined based on the VARC-3 criteria7. For all patients with suspected clinical valve thrombosis, MDCT was performed to confirm reduced leaflet motion and/or hypoattenuated leaflet thickening. Based on the presence or absence of haemodynamic change, SVD was categorised as HVD stage 1 (morphological valve deterioration without haemodynamic change), HVD stage 2 (moderate haemodynamic valve deterioration), or HVD stage 3 (severe haemodynamic valve deterioration). As a patient-oriented clinical endpoint, the clinical consequence of BVD was assessed as BVF and classified as BVF stage 1 (any new-onset or worsening symptoms and signs of pressure/volume overload), BVF stage 2 (valve reintervention), or BVF stage 3 (severe haemodynamic deterioration).
Statistical analysis
Continuous variables were assessed for normal distribution using the Levene test and are presented as the median and interquartile range (IQR; 25%-75%). Dichotomous variables are described as numbers and percentages. The differences in the categorical variables between patients with BVD and those without BVD were compared using the chi-square test or Fisher’s exact test. The differences in the continuous variables between patients with BVD and those without BVD were compared using the Student’s t-test or the Mann-Whitney U test. Exposure-adjusted event rates of BVD, HVD, and BVF were estimated by dividing the number of events reported by the total follow-up time of the study population and reported using a patient-year unit (per 100 patient-years), based on the recommendation of the VARC-3 criteria7. To compare the exposure-adjusted event rates between valve design (balloon-expandable vs self-expanding valve), a Poisson regression model was used, with the number of events as the dependent variable and the log of the exposure time as the offset. To estimate the predictors of BVD, we used the Fine-Gray competing risk regression model to account for the competing risk of death and generated subdistribution hazard ratios (sub-HR). Cox regression analysis was also performed to assess the consistency of our competing risk model. We calculated a crude unadjusted sub-HR using Fine-Gray competing risk regression and then built a multivariate competing risk model that included clinically significant factors. To assess whether the results of the competing risk model were consistent across specific clinical statuses, including small body surface area (≤1.42 m2, defined as the median), small annulus (defined as an annulus area <400 mm2 or perimeter <72 mm12), and valve design (balloon-expandable vs self-expanding valve), we performed a post hoc analysis with interaction testing. Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS), version 25 (IBM) software. The competing risk analysis was performed using Stata version 15.1 (Stata Corp). A p-value <0.05 was considered statistically significant.
Results
Baseline characteristics
In this analysis, 514 patients were included after the exclusion of ineligible patients (Figure 1). Baseline characteristics of the overall population are shown in Table 1. The study population was mainly composed of females with a relatively small body surface area, and more than half of them had chronic kidney disease and hypertension. TAVI was performed via transfemoral access, and almost all TAVI was done for native tricuspid aortic stenosis. Predilatation before valve implantation was performed in nearly 40% of the population, and the rate of TAVI using a balloon-expandable valve was higher than TAVI with a self-expanding valve.
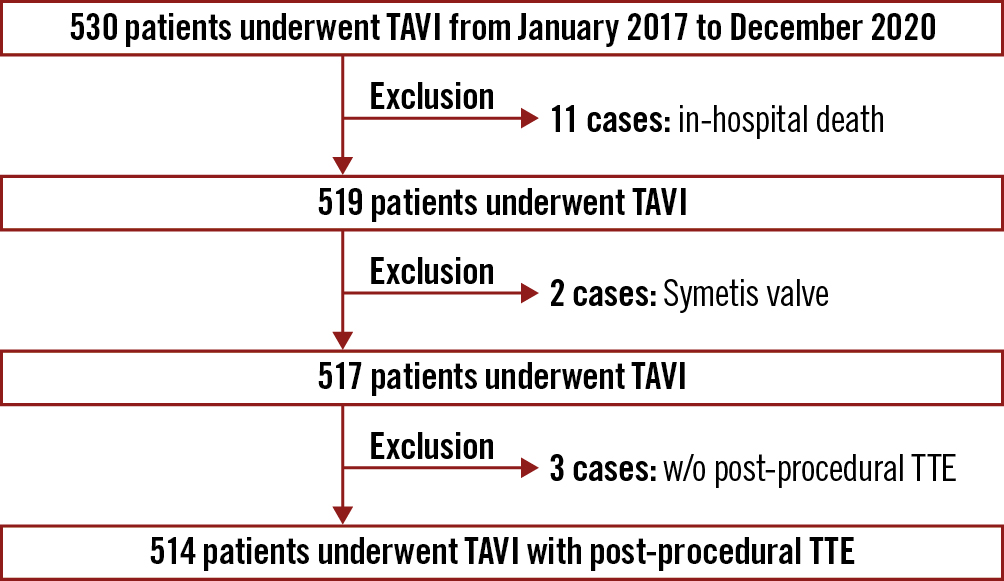
Figure 1. Study population. TAVI: transcatheter aortic valve implantation; TTE: transthoracic echocardiogram; w/o: without
Table 1. Patient characteristics and procedure index.
| Variable | All patients(n=514) |
|---|---|
| Age (years) | 85 (81-88) |
| Male | 158 (30.7%) |
| Body surface area (m2) | 1.42 (1.31-1.56) |
| Body mass index (kg/m2) | 21.9 (19.6-24.7) |
| Clinical frailty scale ≥5 | 77 (15.2%) |
| Hypertension | 398 (77.4%) |
| Diabetes mellitus | 166 (32.3%) |
| Dyslipidaemia | 227 (44.2%) |
| Chronic kidney disease | 336 (65.4%) |
| History of coronary artery disease | 193 (37.5%) |
| History of stroke | 63 (12.3%) |
| Atrial fibrillation on anticoagulation | 109 (21.2%) |
| STS risk score | 5.97 (4.11-8.59) |
| Preprocedural echocardiography | |
| Peak velocity (m/sec) | 4.35 (4.06-4.91) |
| Mean pressure gradient (mmHg) | 44 (35-56) |
| Aortic valve area index (cm2/m2) | 0.46 (0.37-0.56) |
| Left ventricular ejection fraction (%) | 60 (49-64) |
| Computed tomographic findings | |
| Bicuspid | 21 (4.1%) |
| Annulus area (mm2) | 379 (339-429) |
| Diameter of non-coronary cusp (mm) | 30.3 (28.2-32.4) |
| Diameter of right coronary cusp (mm) | 29.1 (27.3-31.0) |
| Diameter of left coronary cusp (mm) | 30.1 (28.2-32.2) |
| Calcium volume (HU-850 threshold) | |
| Total (mm3) | 229.1 (102.6-400.9) |
| Non-coronary cusp (mm3) | 106.1 (40.3-193.9) |
| Right coronary cusp (mm3) | 47.9 (13.8-113.3) |
| Left coronary cusp (mm3) | 40.3 (14.6-98.0) |
| Procedural index | |
| Transfemoral approach | 488 (94.9%) |
| TAVI for failed prosthesis | 11 (2.1%) |
| Non-contrast TAVI | 43 (8.4%) |
| Predilation | 228 (44.4%) |
| Post-dilation | 18 (3.5%) |
| Balloon-expandable valve | 290 (56.4%) |
| Valve size | |
| Balloon-expandable valve 20 mm | 16 (3.1%) |
| Balloon-expandable valve 23 mm | 176 (34.2%) |
| Balloon-expandable valve 26 mm | 81 (15.8%) |
| Balloon-expandable valve 29 mm | 17 (3.3%) |
| Self-expanding valve 23 mm | 37 (7.2%) |
| Self-expanding valve 26 mm | 123 (23.9%) |
| Self-expanding valve 29 mm | 59 (11.5%) |
| Self-expanding valve 34 mm | 5 (1.0%) |
| Data are presented as median (25th-75th percentiles) or numbers (percentages). HU: Hounsfield units; STS: Society of Thoracic Surgeons; TAVI: transcatheter aortic valve implantation | |
Incidence of BVD and BVF
At a median follow-up of 1.9 (IQR 1.9-2.8) years (980.5 patient-years), the rate of BVD was observed in 7.5 events per 100 patient-years (n=74) (Central illustration). Of 74 BVD cases, non-structural valve deterioration, mainly caused by moderate or severe PPM, was observed in 6.3 events per 100 patient-years (n=62). The other components of BVD, in decreasing order, were endocarditis (n=7), SVD (n=3), and clinical valve thrombosis (n=2) (Table 2). Progression to HVD from BVD occurred in 1.2 per 100 patient-years (n=12), and most of them had HVD stage 2 (n=8). Further, the rate of BVF during follow-up was 1.1 per 100 patient-years (n=11). Stage 3 BVF occurred in one patient due to uncontrolled infectious endocarditis. Stage 2 BVF occurred in two patients. In one case, progression to very severe aortic stenosis at 4 years after TAVI required valvuloplasty, whereas another patient with acute decompensated heart failure due to endocarditis required surgical valve replacement. The competing risk regression analysis demonstrated that predilatation was associated with a lower rate of BVD (adjusted sub-HR 0.42, 95% confidence interval [CI]: 0.21-0.88) (Table 3). These results were consistent with the results of the Cox regression analysis (Supplementary Table 1).
Because predilatation may reduce BVD after TAVI, we evaluated further in order to identify the subgroup that might benefit from predilatation. In the subgroup analysis, we found patients with small aortic annuli were likely to benefit from predilatation, while body surface area and differences in valve design did not affect the relationship between predilatation and BVD after TAVI (Figure 2).
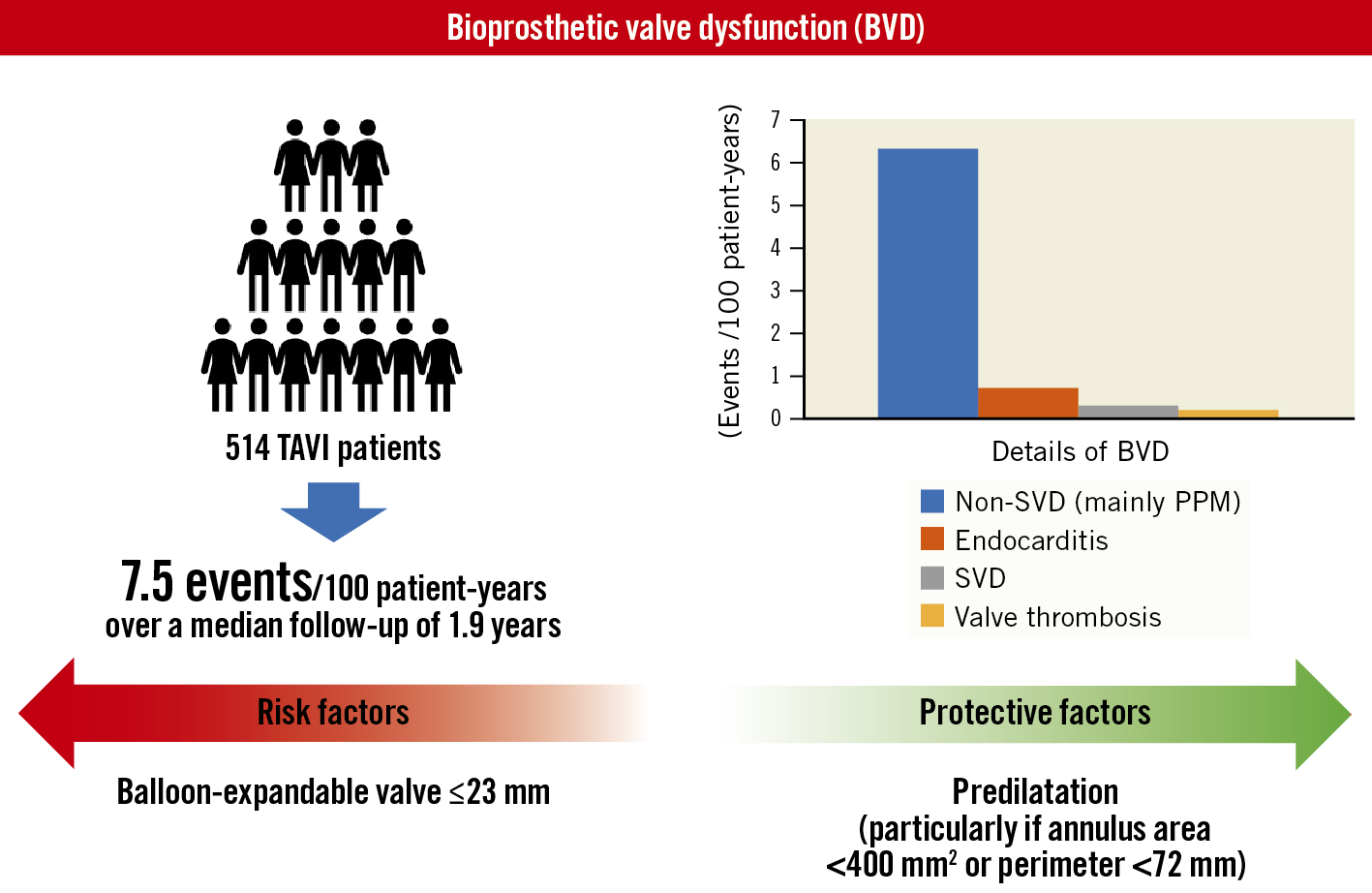
Central illustration. Exposure-adjusted incidence rates, details, and predictors of BVD. PPM: prosthesis-patient mismatch; SVD: structural valve deterioration; TAVI: transcatheter aortic valve implantation
Table 2. Exposure-adjusted event rate and incidence of BVD, HVD, and BVF.
| Exposure-adjusted event rate | No. of events | |
|---|---|---|
| Bioprosthetic valve dysfunction (BVD) | 7.5/100 patient-years | 74 |
| Structural valve deterioration | 0.3/100 patient-years | 3 |
| Non-structural valve deterioration | 6.3/100 patient-years | 62 |
| Moderate prosthesis-patient mismatch | 5.2/100 patient-years | 51 |
| Severe prosthesis-patient mismatch | 0.8/100 patient-years | 8 |
| Paravalvular regurgitation | 0.3/100 patient-years | 3 |
| Clinical valve thrombosis | 0.2/100 patient-years | 2 |
| Hypoattenuated leaflet thickening | 0.2/100 patient-years | 2 |
| Endocarditis | 0.7/100 patient-years | 7 |
| Haemodynamic valve deterioration (HVD) | 1.2/100 patient-years | 12 |
| Stage 1 | 0.2/100 patient-years | 2 |
| Stage 2 | 0.8/100 patient-years | 8 |
| Stage 3 | 0.2/100 patient-years | 2 |
| Bioprosthetic valve failure (BVF) | 1.1/100 patient-years | 11 |
| Stage 1 | 0.8/100 patient-years | 8 |
| Stage 2 | 0.2/100 patient-years | 2 |
| Stage 3 | 0.1/100 patient-years | 1 |
Table 3. Fine-Gray competing risk regression analysis for predicting bioprosthetic valve dysfunction.
| Unadjusted | Adjusted (model 1) | Adjusted (model 2) | ||||
|---|---|---|---|---|---|---|
| Variable | Sub-HR (95% CI) | p-value | Sub-HR (95% CI) | p-value | Sub-HR (95% CI) | p-value |
| Age, years | 0.98 (0.93-1.03) | 0.38 | 0.99 (0.95-1.05) | 0.89 | 0.99 (0.94-1.05) | 0.80 |
| Male | 1.58 (0.98-2.57) | 0.06 | 1.14 (0.58-2.23) | 0.70 | 1.72 (0.86-3.42) | 0.12 |
| Body surface area, m2 | 4.93 (1.35-17.97) | 0.02 | 2.19 (0.31-15.62) | 0.43 | 2.65 (0.39-18.05) | 0.32 |
| Chronic kidney disease | 1.21 (0.72-2.05) | 0.46 | – | – | – | – |
| Atrial fibrillation | 1.30 (0.74-2.26) | 0.36 | – | – | – | – |
| Bicuspid | 1.39 (0.56-3.40) | 0.48 | – | – | – | – |
| Calcium volume | 0.99 (0.99-1.01) | 0.77 | – | – | – | – |
| Small annulus | 0.69 (0.42-1.13) | 0.14 | – | – | – | – |
| TAVI for failed prosthesis | 2.52 (0.79-8.06) | 0.12 | – | – | – | – |
| Predilatation | 0.29 (0.16-0.53) | <0.01 | 0.42 (0.21-0.88) | 0.02 | 0.44 (0.23-0.82) | <0.01 |
| Small annulus without predilatation | 1.74 (1.07-2.84) | 0.03 | – | – | – | – |
| Balloon-expandable valve (BEV) | 2.95 (1.64-5.31) | <0.01 | 1.67 (0.82-3.37) | 0.16 | – | – |
| Valve size 23 mm or less | 2.27 (1.38-3.73) | <0.01 | – | – | – | – |
| BEV 23 mm or less | 2.41 (1.49-3.91) | <0.01 | – | – | 2.46 (1.38-4.38) | <0.01 |
| Self-expanding valve 23 mm | 0.79 (0.28-2.17) | 0.64 | – | – | – | – |
| CI: confidence interval; sub-HR: subdistribution hazard ratio; TAVI: transcatheter aortic valve implantation | ||||||
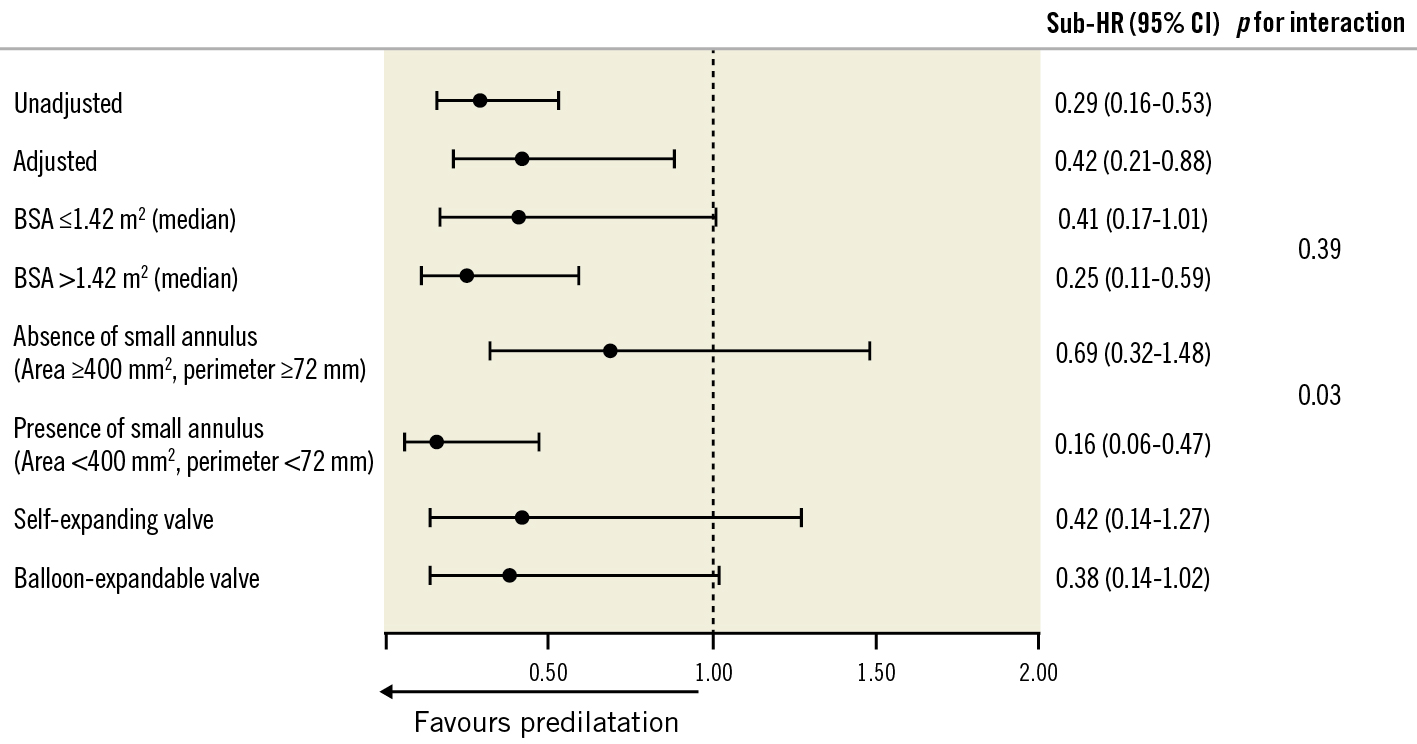
Figure 2. Impact of predilatation on BVD across specific clinical profiles. BSA: body surface area; BVD: bioprosthetic valve dysfunction; CI: confidence interval; Sub-HR: adjusted subdistribution hazard ratio
Exposure-adjusted event rates of BVD according to valve design and size
The rate of BVD was evaluated according to valve design. Of 512 patients, 290 patients underwent TAVI with balloon-expandable valves, and they were likely to have a higher rate of BVD as compared to those with self-expanding valves (10.2 vs 4.0 per 100 patient-years; p<0.01). Among the components of BVD, the rate of non-structural valve deterioration was significantly higher in balloon-expandable valves than that of self-expanding valves (8.6 vs 3.3 per 100 patient-years; p<0.01) (Supplementary Figure 1 and Supplementary Table 2). However, the rates of structural valve deterioration, valve thrombosis, and endocarditis were comparable between balloon-expandable and self-expanding valves (0.3 vs 0 per 100 patient-years, 0.2 vs 0.2 per 100 patient-years, 0.9 vs 0.5 per 100 patient-years, respectively). In evaluating the rate of BVD according to prosthesis size, a small prosthesis size, particularly 23 mm or less, was associated with a higher rate of BVD (Figure 3). Due to the difference in the effective orifice area of balloon-expandable valves and self-expanding valves, we further evaluated the relationship between prosthesis size and the rate of BVD for each valve design. As a result, the association between small prosthesis sizes and high BVD rates was only observed in TAVI with small balloon-expandable valves (≤23 mm). The multivariate competing risk regression analysis confirmed that TAVI with small balloon-expandable valves (≤23 mm) was significantly associated with high rates of BVD (adjusted sub-HR 2.46, 95% CI: 1.38-4.38) (Table 3).
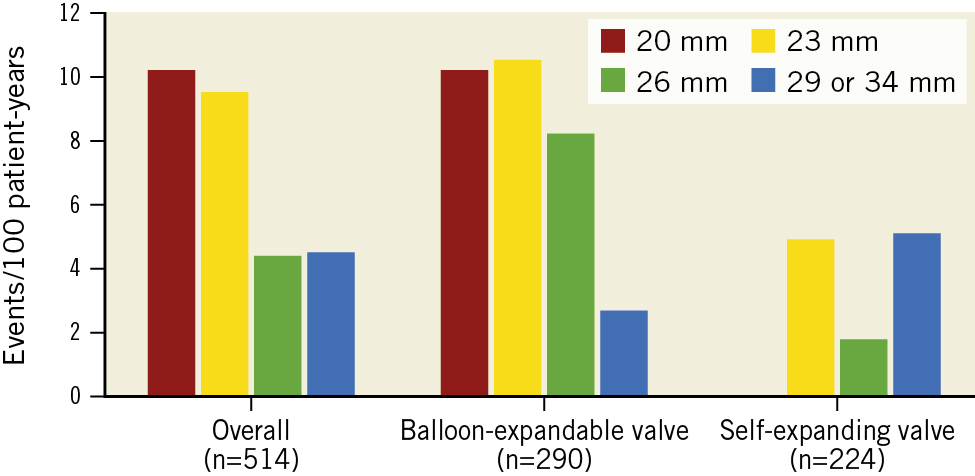
Figure 3. Rate of BVD according to prosthesis size and valve design. BVD: bioprosthetic valve dysfunction
Discussion
The main findings of the current analysis are as follows: 1) BVD was observed in 7.5 events per 100 patient-years for the study population, mainly driven by moderate or severe PPM; 2) the rate of progression from BVD to haemodynamic deterioration and bioprosthetic valve failure was low, 3) predilatation and TAVI with small balloon-expandable valves (≤23 mm) might have an impact on the rate of BVD, which is caused by high rates of moderate or severe PPM. To our knowledge, this is the first report demonstrating the rate of BVD according to the recently updated criteria and the clinical characteristics of BVD after TAVI using the latest-generation medical devices.
Although TAVI has a high procedural success rate and excellent clinical outcomes at short-term follow-up, there is a lack of data on long-term clinical outcomes, including the long-term durability of the bioprosthetic valve. The durability of prosthetic valves is influenced by several factors, including SVD, non-SVD (PPM, paravalvular leakage, thrombosis, and endocarditis), and TAVI-related factors (for example crimping, residual native annular calcification, asymmetric expansion), and it is very important to understand how they mutually interact1314. Several guidelines have advocated varying definitions of valve dysfunction to identify the clinical characteristics of BVD; however, they have not reached a universal consensus151617. The VARC-3 recently proposed updated endpoint definitions of BVD, HVD, and BVF, though the incidence of BVD, according to the VARC-3 criteria, has not been well assessed7. For this reason, this analysis sought to evaluate the incidence and characteristics of BVD, based on the VARC-3 definition, in patients undergoing TAVI using the latest-generation transcatheter aortic valves.
A distinguishing finding of our analysis is that the incidence of BVD in patients undergoing TAVI with the latest-generation prostheses was relatively low (6.7 events per 100 patient-years, 12.8%), mainly due to moderate or severe PPM after TAVI. The incidence of PPM varies depending on the study population, and indeed, the proportion of PPM after TAVI in Western countries is almost double that of the Asian population1819. Though the prognostic impact of PPM after TAVI on mortality or heart failure hospitalisation differs among the study population, the presence of PPM is not an irrelevant matter because it is related to accelerated SVD due to altered valve haemodynamics and mechanical stress2021. At this point, the prediction and prevention of PPM after TAVI might be an issue of concern when we consider a patient’s long-term care plan.
Interestingly, our analysis demonstrated that predilatation reduced the rate of BVD, which was mainly due to moderate or severe PPM, after TAVI. Continuous technical improvements of TAVI have allowed operators to skip the systemic approach using predilatation and a recent clinical trial has demonstrated that TAVI without predilatation is associated with favourable clinical results22. However, because annular and leaflet calcification of the native aortic annulus complex is not removed during TAVI, unlike in surgical aortic valve replacement, the frame of a prosthetic valve is likely to expand asymmetrically, resulting in SVD23. Therefore, predilatation might have a role in preparing a foundation for preventing an under- or malexpansion of the stent frame. Further, in subgroup analysis, the favourable impact of predilatation in reducing the rate of BVD was likely to be observed in patients with small aortic annuli. Based on a previous study demonstrating that a small aortic annulus tends to generate a higher compressive force on the stent frame of a transcatheter valve than a larger annulus, it may be possible that predilatation has the potential to achieve a larger acute gain of aortic annulus diameter, which may result in larger effective orifice area after TAVI24. Moreover, the relationship between small balloon-expandable valves and higher rates of BVD in the current analysis was consistent with the result of a previous study25. Though the valve selection should be based on not only the aortic annuli but the aortic annulus complex and/or the clinical situation, self-expanding valves might have priority, particularly in small aortic annuli.
This analysis demonstrated that the rate of BVD after TAVI using current medical devices was relatively low, and the performance of predilatation was related to a lower risk of BVD caused by moderate or severe PPM. Because the presence of BVD accelerates structural valve deterioration resulting in shortened valve durability, we believed that the appropriate procedural steps, including predilatation and appropriate valve selection, might be important to achieve long-term valve durability. Based on our results, an appropriate procedural algorithm for preventing BVD should start with the assessment of small aortic annuli. If the patient has a small aortic annulus, predilatation should be mandatory and a self-expanding valve might be preferable if the anatomy is suitable. For larger annuli, the prosthesis size could be 26 mm or larger, predilatation might be considered based on the anatomy (i.e., severe calcification) and valve selection might not have any impact on BVD. However, if the choice is between a 23 mm balloon-expandable device or a 23-26 mm self-expanding device, predilatation might be mandatory and the preferred prosthesis is the self-expanding valve. Further study might be required to verify an appropriate procedural algorithm for preventing BVD in current TAVI practice.
Limitations
Our study had several limitations. First, since this was a retrospective analysis of a single-centre observational cohort, inherent methodological limitations which generate bias might have been involved. Second, the procedural steps, including predilatation, and selection of the transcatheter heart valve were at the discretion of the operators. Third, post-procedural CT was not mandatory, and we could not report the rate of subclinical valve thrombosis in this analysis. However, as subclinical valve thrombosis was not associated with prognosis or bioprosthetic valve failure, this analysis did not include subclinical leaflet thrombosis as a factor of BVD26. Fourth, the follow-up duration was not long enough to determine the precise rate of HVD or BVF, because these events may become more clinically relevant in the long-term. Fifth, whether the presence of BVD at midterm follow-up has an impact on valve-oriented and patient-oriented outcomes was not clear. And sixth, due to the nature of observational studies, the result of the subgroup analysis was inconclusive in identifying the ideal population for whom predilatation should be performed before valve implantation. Therefore, further research is required to select the best candidates for predilatation in order to reduce BVD in a randomised population.
Conclusions
Our study suggested that the rate of BVD in patients undergoing TAVI with current medical devices is relatively low at midterm follow-up. Predilatation might have a protective effect for preventing moderate or severe PPM, which was the main contributor to BVD, whereas TAVI with small balloon-expandable valves was a risk factor for BVD. Appropriate procedural steps, including predilatation and/or appropriate prosthesis selection, to mitigate BVD are needed when considering the lifetime management of the TAVI population, particularly for those patients with small aortic annuli.
Impact on daily practice
The recent VARC-3 criteria redefined bioprosthetic valve dysfunction (BVD); this should be taken into account when considering valve durability after transcatheter aortic valve implantation (TAVI). The current analysis showed that the rate of BVD after TAVI using the latest transcatheter valves was relatively low and most of the incidences of BVD were caused by prosthesis-patient mismatch (PPM). Predilatation, particularly in small aortic annuli, had a protective effect in preventing moderate or severe PPM, whereas small balloon-expandable valves increased the risk of BVD, mainly moderate or severe PPM. Though further follow-up is required to evaluate whether BVD is associated with clinical outcomes, our results indicate that following the appropriate procedural steps, including predilatation and appropriate valve selection, may reduce BVD, mainly caused by PPM, after TAVI, particularly in patients with small aortic annuli.
Conflict of interest statement
Y. Watanabe is a clinical proctor for Edwards Lifesciences and Medtronic. The other authors have no conflicts of interest to declare.

