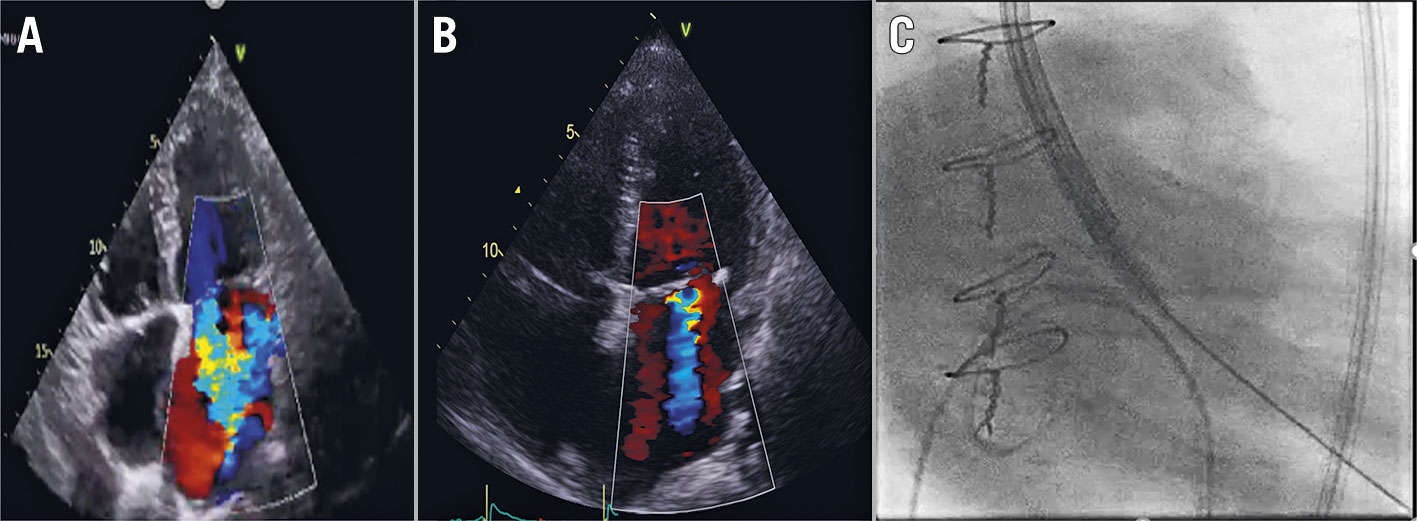
Figure 1. Emergent ViV TAVI. A) Pre-TAVI mitral regurgitation; B) Post-TAVI mitral regurgitation; C) Resuscitation captured on fluoroscopy.
A 69-year-old female presented with a 6-month history of New York Heart Association (NYHA) Functional Class II exertional dyspnoea. Five years prior to the current presentation, she underwent surgical aortic valve replacement (21 mm Trifecta bioprosthesis; Abbott/St. Jude Medical) due to symptomatic severe aortic stenosis with an underlying bicuspid aortic valve. She had a concomitant tricuspid valve repair with a 30 mm MC3 ring (Edwards Lifesciences). A subsequent echocardiogram showed severe stenosis of the aortic valve bioprosthesis (peak velocity: 4.7 ms-1; mean gradient: 51 mmHg; effective orifice area: 0.8 cm2) with a normal left ventricular (LV) function and moderate mitral regurgitation. Coronary angiography did not reveal significant coronary artery disease. She was accepted for redo surgical aortic valve replacement. While waiting for surgery, she deteriorated rapidly within three months to NYHA Class III symptoms. On the day of surgery, the preoperative transoesophageal echocardiogram revealed severe LV dysfunction and severe functional mitral regurgitation, which was confirmed on a transthoracic echocardiogram with a left ventricular ejection fraction (LVEF) of 20% (Figure 1A). The consensus decision was to abandon surgery due to the prohibitive risk and to pursue emergent valve-in-valve transcatheter aortic valve implantation (TAVI).
She progressed rapidly to cardiogenic shock, requiring intensive care admission and vasopressor support. Pre-TAVI evaluation suggested that the risk of coronary obstruction was low (Figure 1B). The valve of choice was a 23 mm Evolut R valve (Medtronic).
Due to her precarious clinical status, the decision was made to proceed with emergent aortic valve-in-valve implantation. Given the immediate availability of definite therapy, the decision was made not to pursue upfront circulatory support, which would have delayed the therapy and complicated vascular access. Under local anaesthetic without sedation, access was obtained in the left femoral artery with a 6 Fr sheath; in the left femoral vein with a 7 Fr sheath; and in the right femoral artery with a 6 Fr sheath which was then upsized to a 14 Fr sheath.
While access was gained, the patient developed acute pulmonary oedema and then respiratory arrest. The patient then progressed to pulseless electrical activity followed by asystolic cardiac arrest. Cardiopulmonary resuscitation was commenced immediately, and she was intubated and then ventilated.
The decision was made to proceed with valve implantation. However, with the interruption of chest compressions, the operators were not able to perform retrograde crossing of the aortic valve despite using multiple catheter shapes and guidewires. It was thought that as there was no cardiac output, the severely restricted aortic leaflets could not open to allow the guidewire to cross.
The operators then decided to cross the valve with chest compression in progress (Figure 1C, Moving image 1). This resulted in successful crossing and subsequently successful valve deployment, with almost immediate haemodynamic recovery. The valve was post-dilated with an 18 mm Tyshak balloon (BVM Medical). The patient was supported with intra-aortic balloon pump insertion. Total downtime was 15 minutes. The on-the-table transoesophageal echocardiogram showed improvement of LV function to an LVEF of 30%. The next-day transthoracic echocardiogram showed a normalised LV function (LVEF of 65%) and moderate mitral regurgitation. The patient had a remarkable clinical recovery with no neurological deficit. At 12 month follow-up, she remained well and independent. Her follow-up echocardiogram showed satisfactory valve-in-valve haemodynamics (mean gradient: 13 mmHg), with preserved LV function (LVEF of 54 %) and only trivial mitral regurgitation.
Conflict of interest statement
K. Poon is a consultant for Edwards Lifesciences and has received institutional research grants from Abbott, Medtronic, and Edwards Lifesciences, and travel support from Edwards Lifesciences and Medtronic. D. Murdoch is a consultant for Edwards Lifesciences and Medtronic. M. Abdul Halim has no conflict of interest to declare.
