Introduction
Multivessel coronary artery disease (CAD) is a common finding in patients with acute coronary syndrome (ACS), affecting approximately 50% of patients with ST-segment elevation myocardial infarction (STEMI)1. This is associated with worse prognosis, increased mortality, and higher costs compared to single-vessel disease12. Despite advancements in therapies and interventional techniques, the presence of multiple lesions continues to pose a clinical challenge for cardiologists3, with great uncertainty regarding the optimal revascularisation strategy. The potential for physiology to guide treatment has garnered increasing interest.
Fractional flow reserve (FFR) is a coronary physiological index measured invasively to determine the potential of a lesion to impede perfusion and induce myocardial ischaemia. It is the ratio between the maximal myocardial blood flow in a stenotic coronary artery and the normal maximal myocardial blood flow in the same artery. Although currently recommended by international guidelines as one of the standard tools to assess the haemodynamic severity of non-culprit lesions (NCLs) in stable CAD456, in patients with ACS concomitant with multivessel disease (MVD), the role and accuracy of FFR to guide revascularisation are less clear. This review summarises current evidence relating to the role of physiology in ACS patients with MVD.
Technical aspects and validation of FFR measurement
During diagnostic cardiac catheterisation, a pressure-sensitive guidewire is advanced into a coronary artery to measure the pressure proximal and distal to a lesion during maximal hyperaemia. This is usually achieved by administering intravenous adenosine, or intracoronary adenosine or papaverine, resulting in vasodilation. FFR is calculated as the ratio of pressure distal to the stenosis (Pd) and pressure proximal to the stenosis (Pa) during maximal hyperaemia7: FFR=Pdhyperaemic/Pahyperaemic7. Lesions with FFR>0.80 (negative FFR) are deemed haemodynamically non-significant, and optimal medical therapy (OMT) is recommended89. Lesions with FFR ≤0.80 (positive FFR) are considered haemodynamically significant, i.e., with the potential to cause ischaemia, and percutaneous coronary intervention (PCI) should be considered alongside OMT91011. The>0.80 cutoff excludes ischaemic lesions with a positive predictive value of 95%; this threshold for guiding PCI has been validated in previous studies7912.
FFR is well established and mandated in stable CAD4. Several landmark trials have validated its accuracy in measuring stenosis severity and its benefit on outcomes81011. For instance, the Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (F.A.M.E.) trial found FFR-guided PCI (FFR ≤0.80) in MVD to be associated with a lower incidence of major adverse cardiovascular events (MACE) for up to 2 years, with fewer stents implanted, compared to angiographic guidance10.
Conversely, non-hyperaemic pressure ratios (NHPRs) are valid wire-based alternatives to FFR that evaluate the functional significance of coronary lesions during the resting Pd/Pa ratio, eliminating the need for vasodilator administration. The instantaneous wave-free ratio (iFR) measures the mean Pd/Pa during the mid-diastolic wave-free period; a window starting from 25% of the way into diastole and continuing until 5 milliseconds before the start of systole13. This provides reliable circumstances for pressure assessment, as coronary microvascular resistance is minimal and constant13. Based on several trials1314151617, iFR is recommended for evaluating intermediate coronary stenoses by the European and the American guidelines for chronic CAD, indicating revascularisation if iFR ≤0.894918. Resting full-cycle ratio (RFR) is another NHPR, representing the smallest Pd/Pa measurement across the entire cardiac cycle19.
Current recommendations for complete revascularisation in ACS patients with MVD
The latest 2023 European ACS guidelines recommend performing complete revascularisation for STEMI patients with MVD (Class I, Level of Evidence A), avoiding the use of functional assessment for the non-infarct related arteries (non-IRAs) during the index procedure20. This is based on large trials establishing its superiority over culprit-lesion-only revascularisation212223. A meta-analysis of 12 randomised controlled trials (RCTs), comparing patient outcomes undergoing multivessel revascularisation or culprit-only PCI for STEMI, found that multivessel revascularisation was associated with lower rates of MACE (by 56%), angina (by 54%), and repeat PCI (by 28%) compared to culprit-only revascularisation24. This was supported by a separate systematic review that included 7,030 patients25. For haemodynamically stable patients with non-ST-segment elevation acute coronary syndrome (NSTE-ACS) and MVD, European guidelines recommend consideration of complete revascularisation (Class IIa, Level of Evidence C)20, and invasive physiology should be considered to assess non-IRAs2026.
The optimal strategy to decide which NCLs to treat remains subject to ongoing debate20. Visual assessment is reported to overestimate stenosis severity, particularly intermediate stenoses (50-70% diameter stenosis)27, which may lead to overtreatment of lesions that cause neither ischaemia nor symptoms, thus exposing patients to unnecessary risks28293031. FFR is not frequently used in this setting partly owing to concerns of microvascular disturbance during the acute phase of a myocardial infarction (MI), which may attenuate hyperaemic response to vasodilators and, thus, impair FFR reliability32333435. Despite this, several trials have evaluated the application of FFR in this patient cohort, showing promising results3637.
FFR use in guiding PCI of non-culprit lesions for ACS patients with MVD
To date, the Complete vs Culprit-only Revascularization to Treat Multi-vessel Disease After Early PCI for STEMI (COMPLETE) trial is the largest study addressing complete revascularisation in ACS patients with MVD. Among 4,041 STEMI patients with MVD, it found that complete revascularisation of significant NCLs (n=2,016) was superior to culprit-only revascularisation (n=2,025) in reducing hard clinical endpoints over a 3-year follow-up23. This includes a 26% risk reduction for a composite of cardiovascular mortality or new MI in the group assigned complete revascularisation, driven by a 32% lower incidence of new MI23. Incidence of the co-primary composite endpoint – comprising cardiovascular death, new MI, or ischaemia-driven revascularisation – was similarly lower in the complete revascularisation group, by roughly 50%23. Yet, no reduction in heart failure or all-cause mortality was observed23. Secondary analysis of the trial also observed more angina-free individuals by the end of the study in the group assigned complete revascularisation38. It should be noted that in the COMPLETE trial, physiology was not used alone to guide complete revascularisation; NCLs were deemed significant if they presented with either stenosis ≥70% of vessel diameter on angiographic visual estimation or FFR ≤0.80 with 50-69% stenosis23. While FFR was not standardised for all patients, the positive results furthered interest into the potential benefits of an FFR-guided approach in this cohort.
Several trials have directly compared FFR-guided complete PCI to culprit-only PCI in ACS patients with MVD. Engström et al randomised 627 patients with STEMI and MVD to either FFR-guided complete revascularisation or culprit-only PCI. Those assigned FFR-guided complete revascularisation had a significantly lower risk of a composite of all-cause mortality, non-fatal reinfarction, and ischaemia-driven revascularisation, compared to the culprit-only group (hazard ratio [HR] 0.56, 95% confidence interval [CI]: 0.38-0.83; p=0.004)30. Importantly, 31% of the patients allocated to complete revascularisation did not undergo revascularisation of NCLs, as their FFR values were>0.8030. This did not cause significant differences in the primary outcome rates compared to the remainder of the group assigned complete revascularisation (HR 1.54, 95% CI: 0.82-2.90; p=0.180)30.
Similarly, the Comparison Between FFR Guided Revascularization Versus Conventional Strategy in Acute STEMI Patients With MVD (CompareAcute) trial supports the superiority of FFR-guided complete revascularisation compared to culprit-only PCI in STEMI patients with MVD. Among 885 patients, FFR-guided PCI of NCLs lowered the risk of a composite of major adverse cardiovascular and cerebrovascular events, including all-cause mortality, MI, revascularisation, and cerebrovascular events, compared to no additional invasive treatment besides primary PCI (pPCI), both at 1-year and 3-year follow-up (p<0.001)3139. The primary outcomes in these 2 trials were mainly driven by fewer repeat revascularisations in patients assigned complete revascularisation3139, and they failed to show any differences in mortality or non-fatal MI, albeit neither trial was sufficiently powered to identify differences in hard clinical endpoints, i.e., mortality and MI303139. From an economic standpoint, an FFR-guided approach is favourable. Cost analyses from the CompareAcute trial demonstrate a decrease in healthcare costs using an FFR-guided complete revascularisation strategy by up to 21% (at 1 year) and 22% (at 3 years), compared to culprit-only PCI31.
Physiology use to guide PCI in older ACS patients with MVD
Over the past decades, clinical research assessing FFR-guided PCI in ACS patients with MVD largely included younger patients, with a paucity of data representing patients aged ≥75 years. However, older adults are disproportionately affected by ACS, experiencing higher rates of complications and MACE40, and often receive suboptimal treatment414243. A subanalysis of patients aged ≥75 years from the DANAMI-3-PRIMULTI trial found no significant difference in MACE with FFR-guided complete revascularisation44. While these findings oppose the FFR-associated prognostic benefit in the full cohort, the small sample size (n=110) prevents any reliable conclusions from being drawn30.
A recent, large RCT addressing the effectiveness of physiology-guided PCI in older patients is the Functional Assessment in Elderly MI Patients With Multivessel Disease (FIRE) trial, wherein 1,445 patients aged ≥75 years, with MVD and either STEMI or non-STEMI (NSTEMI), were randomised to physiology-guided complete revascularisation or culprit-only PCI45. In the former group, 50.1% patients received revascularisation for NCLs, based on physiological assessment comprising guidewire-based methods and quantitative flow ratio (QFR)45. Findings show the superiority of the physiology-guided complete approach over culprit-only PCI in terms of a 27% relative risk reduction in a composite of mortality, stroke, MI, or ischaemia-driven revascularisation45. This was driven by a reduction in each component of the composite endpoint, excluding stroke. Safety was also assessed as a composite of contrast-associated acute kidney injury, stroke, or bleeding, for which no difference was found between the 2 groups (HR 1.11, 95% CI: 0.89-1.37; p=0.370)45. Hence, the demonstrated feasibility, safety, and effectiveness of physiology-guided complete PCI support the potential inclusion of this strategy into routine practice for older adults with ACS and MVD.
Recent contrasting findings
Findings from the Ffr-gUidance for compLete Non-cuLprit REVASCularization (FULL REVASC) trial are controversial. This registry-based RCT randomised 1,542 patients (mean age 65.3±10.5 years) with STEMI or very high-risk NSTEMI and MVD to undergo either FFR-guided complete or culprit-only PCI46. As opposed to most other trials, FULL REVASC showed that compared to culprit-only PCI, FFR-guided complete revascularisation did not cause a significant difference in the primary composite outcome – comprising all-cause death, MI, or unplanned revascularisation – at 4.8 years (HR 0.93, 95% CI: 0.74-1.17; p=0.530)46. When evaluating how applicable these results are and reasons for this discordance, the following should be acknowledged. The trial aimed to enrol 4,052 patients with a primary endpoint of a composite of all-cause death or MI at 1 year46. Based on feasibility and ethical grounds, it was terminated prematurely with 1,542 patients randomised, hence the addition of unplanned revascularisation to the primary outcome46. Despite the longer follow-up, the 74% statistical power achieved at 4.8 years was lower than expected46. Extrapolating findings to very high-risk NSTEMI patients may not be reliable, as this subgroup constituted only 8.6% of the 1,542 patients enrolled36. Additionally, differences in procedural characteristics could have contributed to the inconsistencies with other trials: the FIRE and COMPLETE trials randomised patients no later than 48 hours45 and 72 hours of successful PCI of the culprit vessel23, respectively, whereas FULL REVASC patients were randomised within 6 hours46. Possible microvasculature disturbance in the hyperacute phase could have overestimated stenosis severity, leading to overtreatment35. However, 18.8% of all the NCLs in the complete revascularisation group were treated with PCI46 − a lower percentage than in other trials (PCI was performed in 45.5% of NCLs in the complete revascularisation arm of the FIRE trial)45, indicating other factors could be at play. Furthermore, with the release of the conclusive COMPLETE trial findings, few patients with severe stenosis or three-vessel disease were included in the FULL REVASC trial3646. Since this cohort benefits substantially from NCL revascularisation, the lack of their representation may have attenuated the overall results.
Angiography-guided PCI versus FFR-guided PCI
Another salient question is whether FFR-guided or angiography-based complete revascularisation is superior. Two major studies comparing these strategies in STEMI patients reveal contrasting results. The FLOWER-MI trial observed that, in 1,171 STEMI patients with MVD, FFR-guided complete revascularisation of NCLs was not superior to angiography-guided complete revascularisation in terms of the 1-year composite risk of death, MI, or urgent revascularisation (p=0.310)47. This insignificant difference was similarly observed in the 3-year follow-up extension phase48, with fewer stents and PCI used in the FFR group. The wide confidence interval for the primary outcome prevents firm conclusion from being drawn.
Conversely, the more recent FRAME-AMI study – enrolling 562 patients with acute MI (STEMI or NSTEMI) and MVD – showed the superiority of FFR-guided PCI of non-IRAs over angiographic guidance, associated with a reduction in death, MI, or repeat revascularisation at a median 3.5-year follow-up (p=0.003)49. This benefit, driven by the outcomes of NSTEMI patients, was consistent regardless of non-IRA stenosis severity49.
These findings should be interpreted cautiously for multiple reasons. Firstly, both trials had insufficient statistical power owing to a low incidence of primary outcome events (54 in FLOWER-MI; 52 at 1 year and 56 at 3 years in FRAME-AMI)474849. The premature termination of FRAME-AMI might have led to exaggerated outcomes, highlighting the need for larger sample sizes. Secondly, of these 2 trials, only FRAME-AMI enrolled NSTEMI patients, making generalisations to this cohort less reliable3649. Thirdly, the FLOWER-MI FFR-guided group, despite undergoing fewer interventions, had 3 times more periprocedural-related MIs than the angio-guided group, potentially explaining why the FFR group had a numerically higher incidence of non-fatal MI474849. Furthermore, reliable comparisons cannot be made between the 2 trials, as the population evaluated, follow-up periods, and timing of non-IRA PCI differ.
Concerning NSTE-ACS patients with MVD, the FAMOUS NSTEMI trial supports the benefit of FFR-guided complete revascularisation compared to angiographic guidance. The former strategy resulted in fewer stents implanted and, while the rate of procedure-related MI was higher in the angiography-guided group and spontaneous MI higher in the FFR-guided group, overall health outcomes were not significantly different50.
The impact of pattern distribution of CAD on post-PCI FFR
In patients with ACS and MVD, the role of coronary physiology may go beyond the definition of the haemodynamic lesion severity and include additional evaluation of the functional atherosclerotic pattern of NCLs. Functional patterns of CAD can be classified into focal, diffuse and mixed patterns according to the distribution of atherosclerotic plaques along the epicardial vessel; these classifications may have an impact on the final procedural results. Focal CAD is usually characterised by a higher plaque burden, mainly containing lipidic components with a high prevalence of thin-cap fibroatheroma, whereas diffuse disease has a higher prevalence of calcifications, leading to plaque stability51. The possibility of stratifying the pattern of CAD to predict the potential benefit of revascularisation has both clinical and prognostic implications. Previous studies have shown that PCI in patients with focal disease results in a larger FFR improvement, higher post-PCI FFR value, reduced ischaemia, and reduced angina compared to patients with diffuse disease receiving PCI5253. Among patients characterised by non-invasive assessment of coronary atherosclerotic distribution, those with diffuse disease undergoing PCI have a significantly higher risk of target vessel failure compared to those with predominant focal lesions54. Therefore, physiology-guided classification of CAD patterns before proceeding to intervention may allow better patient selection and may improve postprocedural outcomes.
While FFR measurement is performed to establish the functional significance of haemodynamic lesions, it does not provide information on the localisation of the pressure gradient loss along the epicardial vessel. To address this limitation, an additional wire pullback has been introduced to supplement the functional assessment by providing information on the longitudinal distribution of pressure drops. The pullback pressure gradient (PPG) is an index defining different patterns of pressure loss on a continuous scale ranging from 0 (diffuse pattern) to 1 (focal pattern). From a practical perspective, PPG calculation can be incorporated into the same procedure as the FFR assessment by performing a manual pullback which takes an additional 30 seconds compared to the standard procedure (Figure 1)55. PPG is then computed using 2 pullback-derived parameters: the maximal pressure difference over 20% of the pullback time and the extent of functional disease. The prospective, large-scale, multicentre PPG Global Registry established the capacity of PPG to predict optimal procedural results and outcomes in patients with stable CAD or who had experienced ACS with MVD. Vessels with focal disease (defined by a PPG cutoff>0.62) treated with PCI achieved significantly higher final FFR values and a larger FFR increase compared to those with diffuse disease treated with PCI. PPG accurately predicted post-PCI FFR value ≥0.88 with an area under the curve (AUC) of 0.82 (95% CI: 0.79-0.84), and the optimal PPG cutoff was 0.73. Conversely, FFR alone did not predict revascularisation outcomes (AUC 0.54, 95% CI: 0.50-0.57)56. In addition, patients with focal disease reported greater physical limitation, worse anginal symptoms, and a lower quality of life compared to patients with diffuse disease56. Thus, the PPG value allows operators to identify subjects who would benefit from revascularisation and those who would incur a suboptimal post-PCI result, influencing the decision-making approach and diverting patients from PCI towards treatment with alternative strategies. This may help to avoid unnecessary invasive treatment in case of a small, expected postprocedural benefit. In the specific setting of ACS patients with MVD, medical therapy could represent the correct initial approach to adopt for the management of NCLs with a pattern of diffuse disease, switching to PCI only in case of persistent symptoms despite optimised medical treatment.
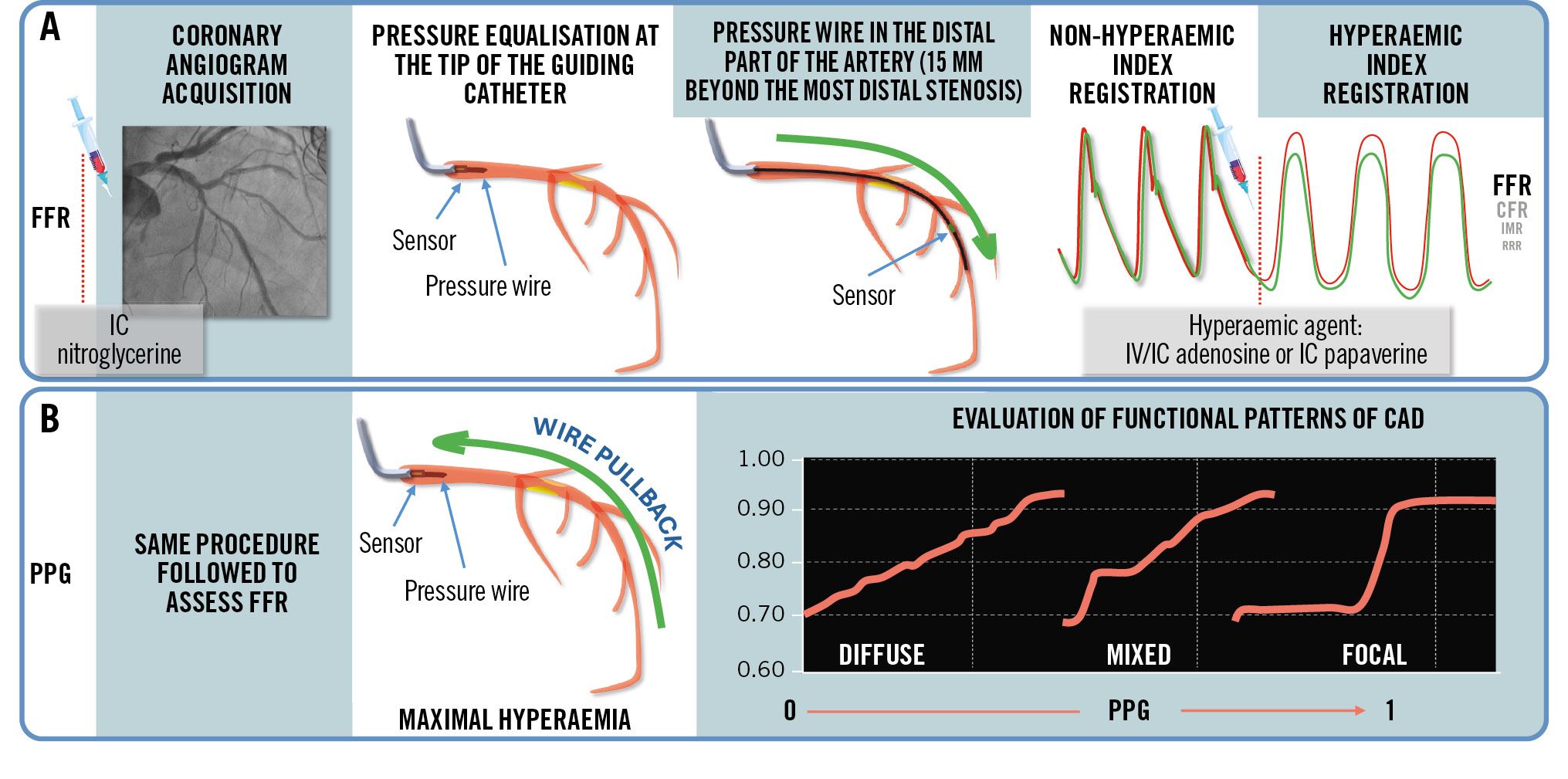
Figure 1. Procedural steps to assess FFR and PPG indices. A) Steps to assess FFR. B) Steps to assess PPG. CAD: coronary artery disease; CFR: coronary flow reserve; FFR: fractional flow reserve; IC: intracoronary; IMR: index of microcirculatory resistance; IV: intravenous; PPG: pullback pressure gradient; RRR: resistive reserve ratio
How to integrate FFR with intracoronary imaging
A physiology-based decision adopted to perform or defer PCI in NCLs is safe and effective in reducing future adverse events compared to an angiography-guided strategy57. However, FFR carries limitations in terms of detecting suboptimal results after stent implantation, such as edge dissection and strut underexpansion and/or malapposition. In a population stratified according to the use of an imaging-guided PCI, Ahn et al recently showed that the post-stenting FFR lost its significant prognostic value in predicting cardiac events at 5 years when optimal results were obtained using an imaging-guided strategy58. In addition, deferred coronary revascularisation based on FFR may be limited, because coronary physiology does not identify a functionally silent vulnerable plaque, which has been associated with a risk of recurrent cardiovascular events5960.
Recent evidence has raised concerns regarding deferred revascularisation based entirely on physiological assessment, especially in some specific populations such as patients with diabetes mellitus, for whom ischaemia is not the only predictor of future adverse events59. In the PREVENT trial, treatment of non-flow-limiting (FFR>0.80) vulnerable plaques with a preventive PCI strategy reduced the composite risk of death from cardiac causes, target vessel MI, ischaemia-driven target vessel revascularisation, or hospitalisation for unstable or progressive angina at 2-year follow-up, compared with OMT alone. Preventive PCI also diminished the patient-oriented composite risk, comprising all-cause death, MI, or any repeat revascularisation60. The randomised FORZA trial had already investigated the use of optical coherence tomography (OCT) or FFR guidance in patients with angiographically intermediate coronary lesions and showed a borderline significant reduction (p=0.048) in the combined occurrence of MACE and residual angina in the OCT arm compared to the FFR arm61.
FFR and intracoronary imaging complement each other, addressing different questions. Applying the use of intracoronary intravascular ultrasound or OCT to patients with non-flow-limiting plaques detected in the functional evaluation could pave the way to improving their characterisation and guidance for revascularisation, especially in cases of a borderline FFR value or ambiguous culprit lesions in the setting of NSTE-ACS. The sole application of coronary physiology in the early phase of an acute context risks underestimating the severity of the lesions since the response to hyperaemic agents may be suboptimal because of the coronary microvascular dysfunction (Table 1). However, some studies investigating the diagnostic accuracy and temporal variation of FFR in NCLs demonstrated good reproducibility between the acute and subacute phases (Table 2). The combined use of imaging and physiology is fundamental in high-risk categories such as diabetic patients, who benefit from early invasive treatment guided by plaque morphology as well as aggressive secondary prevention62.
Novel computational approaches to derive FFR from intracoronary imaging have been recently proposed. The diagnostic performance of the OCT-based FFR (OFR) was evaluated by Yu et al. When compared with standard pressure wire-based FFR, OFR showed good correlation and agreement in a population with intermediate coronary stenoses63. The recent FUSION study is the largest multicentre study comparing OCT-derived physiology (virtual flow reserve [VFR]) with invasive FFR. VFR is obtained through a model that calculates pressure loss along the vessel with a computation time similar to conventional OCT acquisition, facilitating and diverting the choice of treatment in a substantial proportion of patients compared to angiography and imaging-guided PCI without physiology64.
Table 1. Causes of incorrect FFR estimation and their respective mechanisms.
| Cause of incorrectFFR estimation | Reason |
|---|---|
| Early phase of ACS | • Underestimation of the lesion severity due to infarct-related coronary bed dysfunction, which may blunt the maximal hyperaemic response |
| Aortic stenosis | • Blunted effect of adenosine to increased coronary flow, due to vasodilation at rest to avoid subendocardial ischaemia, caused by a combination of the following:- valve stenosis- myocardial hypertrophy with augmented cardiac work- potential CMD |
| Coronary microvascular dysfunction | • An epicardial stenosis may result in less flow limitation in case of CMD due to an increased resistance in the coronary microcirculation affecting the response of the coronary bed to adenosine• IMR ≥25 is an independent predictor of disagreement between RFR and FFR |
| Vasodilator tolerance | • Stimulants such as caffeine antagonise the pharmacological action of adenosine by competitively blocking adenosine receptors activity, potentially causing false-negative measurements |
| ACS: acute coronary syndrome; CMD: coronary microvascular dysfunction; FFR: fractional flow reserve; IMR: index of microcirculatory resistance; RFR: resting full-cycle ratio | |
Table 2. Studies investigating the diagnostic accuracy and temporal variation of FFR and iFR in patients with acute coronary syndrome and multivessel disease.
| Study | Index used | Design | Populations and NCLs | Underestimation of lesion severity based on index | No differences in index values between acute and subacute phase | Results |
|---|---|---|---|---|---|---|
| Ntalianis et al201080 | FFR | Prospective observational | 75 STEMI26 NSTEMI 112 NCLs | Data not applicable | Yes | FFR after pPCI vs FFR after 35±4 days: 0.77±0.13 vs 0.77±0.13; p=NS |
| Musto et al201733 | FFR | Prospective observational | 50 STEMI66 NCLs | Data not applicable | Yes | FFR after pPCI vs FFR after 5-8 days: 0.82±0.07 vs 0.82±0.08; p=0.620 |
| Choi et al 201834 | FFR | Prospective observational | 34 STEMI66 NSTEMI 128 NCLs | Data not applicable | Data not applicable | FFR in STEMI vs FFR in stable angina for 60-70% stenosis:0.81±0.09 vs 0.70±0.12; p=0.285 |
| Van der Hoeven et al 201935 | FFR | Substudy of the REDUCE-MVI RCT | 73 STEMI73 NCLs | Yes | Data not applicable | FFR after pPCI vs FFR after 1 month: 0.88±0.07 vs 0.86±0.09; p=0.001 |
| Mejía-Rentería et al 201981 |
FFR | Multicentric observational | 49 ACS59 NCLs | Data not applicable | Data not applicable | FFR in ACS vs FFR in stable angina: 0.79±0.11 vs 0.80±0.13; p=0.527 |
| Musto et al 201733 | iFR | Prospective observational | 50 STEMI66 NCLs | Data not applicable | Yes | iFR after pPCI vs iFR after 5.9±1.5 days: 0.90±0.06 vs 0.89±0.07; p=0.640 |
| Indolfi et al 201582 | iFR | Prospective observational | 53 ACS78 NCLs | Data not applicable | Data not applicable | iFR in ACS vs iFR in stable CAD: 0.94 (IQR 0.07) vs 0.96 (IQR 0.12); p=NS |
| Thim et al201783 | iFR | Prospective observational | 120 STEMI 157 NCLs | Data not applicable | Yes | iFR after pPCI vs iFR after 16 days (IQR 5-32): 0.89 (IQR 0.82-0.94) vs 0.91 (IQR 0.86-0.96); p=NS |
| Choi et al201834 | iFR | Prospective observational | 34 STEMI66 NSTEMI128 NCLs | Data not applicable | Data not applicable | iFR in STEMI vs iFR in stable IHD for 60-70% stenosis: 0.87±0.08 vs 0.87±0.12; p=0.990 |
| ACS: acute coronary syndrome; FFR: fractional flow reserve; iFR: instantaneous wave-free ratio; IHD: ischaemic heart disease; IQR: interquartile range; NCL: non-culprit lesion; NS: non-significant; NSTEMI: non-ST-segment elevation myocardial infarction; pPCI: primary percutaneous coronary intervention; RCT: randomised controlled trial; REDUCE-MVI: Reducing Micro Vascular Dysfunction in Acute Myocardial Infarction by Ticagrelor; STEMI: ST-segment elevation myocardial infarction | ||||||
FFR versus novel physiology-based assessment tools
Limitations undermine the uptake of FFR into routine practice; these include costs, risks associated with administering pharmacological agents to induce maximal hyperaemia, and an extended procedural time. Novel physiology-based indices have emerged to help overcome these, facilitating assessments among interventional cardiologists.
Several RCTs have validated iFR, showing a diagnostic accuracy similar to FFR and non-inferior clinical outcomes of complete PCI guided by iFR ≤0.89 compared to FFR ≤0.80 for MACE at 1, 2, and 5 years1415161765. A recent substudy has shown the safety of deferring revascularisation based on iFR is comparable to that based on FFR65. However, discrepancies between iFR and FFR occur in about 20% of cases151766. Possible predictors of these discordances include patient sex, age, haemoglobin level, smoking, and renal insufficiency67. While data in ACS patients are limited, some evidence supports the diagnostic accuracy, feasibility, and safety of iFR assessment in STEMI patients with MVD68 (Table 2). Research surrounding other NHPRs remains lacking, though RFR was found to have a high diagnostic accuracy with iFR and concordance with FFR19.
Moreover, advancements in computational flow dynamics and three-dimensional technology have enabled the development of invasive functional coronary angiography, known as angiography-derived FFR. This tool assessing coronary physiology eliminates the need for an invasive pressure wire and drug-induced hyperaemia, and enables online and offline estimation of FFR from angiography. QFR, based on coronary angiography reconstruction and flow velocity calculated by frame count, has shown substantial clinical evidence regarding its diagnostic accuracy and prognostic value. A patient-data meta-analysis of 819 patients and 969 vessels (inclusive of FAVOR Pilot, WIFI II, FAVOR II China, and FAVOR II Europe-Japan trials) demonstrated an overall agreement of 87% between QFR and FFR, with a diagnostic sensitivity and specificity of 84% and 88%, respectively69. The FAVOR III China Study established QFR-guided coronary artery revascularisation to be comparable to FFR-guided PCI70. The effectiveness of QFR-guided PCI is further supported by a subanalysis from the FIRE trial, which also validates the threshold QFR ≤0.80 in identifying vessels at high risk for adverse events71. Similarly, the AQVA trial found a significant improvement in post-PCI physiological results for QFR-guided virtual revascularisation as compared to conventional angiographic guidance72.
The Murray law-based μQFR index enables FFR derivation using a single angiographic projection for the vessel model. Small-scale research showed that its assessment is concordant with three-dimensional QFR73 and FFR74. Other angio-based parameters, such as FFRangio75 and vFFR76, use aortic pressures to determine boundary conditions and have shown promising diagnostic performance. However, the accuracy of angio-based tools is highly dependent on projection quality, angles, and the operator’s technical skills, which may hinder reproducibility77.
Computed tomography (CT)-derived fractional flow reserve (FFR-CT) is a physiological simulation technique that models coronary vessel flow from coronary CT angiography. FFR-CT provides valuable information on the anatomy and coronary physiology of MVD patients, aiding revascularisation decision-making. The ADVANCE Registry showed that FFR-CT modified the treatment strategy in two-thirds of patients with clinically suspected CAD and atherosclerosis, with less invasive coronary angiography at 1 year for those with FFR-CT>0.80 compared to FFR-CT <0.8078. Complex coronary artery lesions can be more accurately assessed by FFR-CT to decide between PCI and coronary artery bypass grafting, beyond relying solely on the SYNTAX score79.
Table 2. Studies investigating the diagnostic accuracy and temporal variation of FFR and iFR in patients with acute coronary syndrome and multivessel disease.
| Study | Index used | Design | Populations and NCLs | Underestimation of lesion severity based on index | No differences in index values between acute and subacute phase | Results |
|---|---|---|---|---|---|---|
| Ntalianis et al201080 | FFR | Prospective observational | 75 STEMI26 NSTEMI 112 NCLs | Data not applicable | Yes | FFR after pPCI vs FFR after 35±4 days: 0.77±0.13 vs 0.77±0.13; p=NS |
| Musto et al201733 | FFR | Prospective observational | 50 STEMI66 NCLs | Data not applicable | Yes | FFR after pPCI vs FFR after 5-8 days: 0.82±0.07 vs 0.82±0.08; p=0.620 |
| Choi et al 201834 | FFR | Prospective observational | 34 STEMI66 NSTEMI 128 NCLs | Data not applicable | Data not applicable | FFR in STEMI vs FFR in stable angina for 60-70% stenosis:0.81±0.09 vs 0.70±0.12; p=0.285 |
| Van der Hoeven et al 201935 | FFR | Substudy of the REDUCE-MVI RCT | 73 STEMI73 NCLs | Yes | Data not applicable | FFR after pPCI vs FFR after 1 month: 0.88±0.07 vs 0.86±0.09; p=0.001 |
| Mejía-Rentería et al 201981 |
FFR | Multicentric observational | 49 ACS59 NCLs | Data not applicable | Data not applicable | FFR in ACS vs FFR in stable angina: 0.79±0.11 vs 0.80±0.13; p=0.527 |
| Musto et al 201733 | iFR | Prospective observational | 50 STEMI66 NCLs | Data not applicable | Yes | iFR after pPCI vs iFR after 5.9±1.5 days: 0.90±0.06 vs 0.89±0.07; p=0.640 |
| Indolfi et al 201582 | iFR | Prospective observational | 53 ACS78 NCLs | Data not applicable | Data not applicable | iFR in ACS vs iFR in stable CAD: 0.94 (IQR 0.07) vs 0.96 (IQR 0.12); p=NS |
| Thim et al201783 | iFR | Prospective observational | 120 STEMI 157 NCLs | Data not applicable | Yes | iFR after pPCI vs iFR after 16 days (IQR 5-32): 0.89 (IQR 0.82-0.94) vs 0.91 (IQR 0.86-0.96); p=NS |
| Choi et al201834 | iFR | Prospective observational | 34 STEMI66 NSTEMI128 NCLs | Data not applicable | Data not applicable | iFR in STEMI vs iFR in stable IHD for 60-70% stenosis: 0.87±0.08 vs 0.87±0.12; p=0.990 |
| ACS: acute coronary syndrome; FFR: fractional flow reserve; iFR: instantaneous wave-free ratio; IHD: ischaemic heart disease; IQR: interquartile range; NCL: non-culprit lesion; NS: non-significant; NSTEMI: non-ST-segment elevation myocardial infarction; pPCI: primary percutaneous coronary intervention; RCT: randomised controlled trial; REDUCE-MVI: Reducing Micro Vascular Dysfunction in Acute Myocardial Infarction by Ticagrelor; STEMI: ST-segment elevation myocardial infarction | ||||||
Future perspectives
With promising evidence, there is a strong potential for FFR to assist decision-making in the management of patients with ACS and MVD. However, its reliability may be limited in acute phase MIs, due to microvascular disturbance, as well as in identifying vulnerable plaques. This dilemma emphasises the need to supplement FFR with intracoronary imaging modalities like OCT, for which several ongoing trials will provide valuable insights. The COMPLETE-2 trial (ClinicalTrials.gov: NCT05701358) aims to enrol 5,100 patients with STEMI or NSTEMI and MVD to compare physiology-guided and angiography-guided approaches to achieve complete revascularisation, providing more definitive conclusions regarding the usefulness of FFR/RFR/iFR in ACS patients with MVD. Findings from the COMPLETE-2 OCT substudy will address the feasibility of combining FFR with intracoronary imaging and its impact on clinical outcomes.
In addition, functional angiography-based indices may overcome some limitations of FFR, particularly in deferring revascularisation in ACS patients with MVD. Ongoing trials (Table 3) are exploring these indices further. FFR-CT is similarly expected to play an important role in NSTE-ACS patients. Additionally, findings from ongoing trials investigating the optimal timing of complete revascularisation in ACS patients with MVD are highly anticipated (Table 4).
Table 3. Ongoing studies investigating novel angiography-based physiology assessment tools.
| Study name | Number of patients | Strategy | Comparator | Primary endpoint | Follow-up |
|---|---|---|---|---|---|
| FAVOR III Europe Japan (NCT03729739) | 2,001 | QFR-based diagnostic strategy | FFR-based diagnostic strategy | Composite of all-cause mortality, any MI, and any unplanned revascularisation | 12 months |
| LIPSIASTRATEGY (NCT03497637) | 1,926 | vFFR | FFR-guided therapy | Composite of cardiac death, non-fatal MI, or unplanned revascularisation | 12 months |
| FAST III (NCT04931771) | 2,228 | Three-dimensional angio-based vFFR-guided revascularisation | FFR-guided revascularisation | Composite of all-cause death, any MI, or any revascularisation | 12 months |
| FLASH FFR II (NCT04575207) | 2,132 | caFFR | FFR-guided revascularisation | Composite of all-cause death, MI, and unplanned revascularisation | 12 months |
| ALL-RISE (NCT05893498) | 1,924 | FFRangio-guided revascularisation | Pressure wire-based guided revascularisation (FFR or NHPR) | Composite of all-cause death, MI, or unplanned clinically driven revascularisation | 12 months |
| ALL-RISE: Advancing Cath Lab Results With FFRangio Coronary Physiology Assessment; caFFR: coronary angiography-derived fractional flow reserve; FAST III: Fractional Flow Reserve or 3D-Quantitative-Coronary-Angiography Based Vessel-FFR Guided Revascularization; FAVOR III Europe Japan: Comparison of Quantitative Flow Ratio (QFR) and Conventional Pressure-wire Based Functional Evaluation for Guiding Coronary Intervention. A Randomized Clinical Non-inferiority Trial; FFR: fractional flow reserve; FFRangio: angiography-derived fractional flow reserve; FLASH FFR II: A Prospective, Multicenter, Blinded, Randomized, Noninferiority Clinical Trial of Coronary Angiography Fractional Flow Reserve (caFFR) Versus Fractional Flow Reserve (FFR) to Guide Percutaneous Coronary Intervention; LIPSIASTRATEGY: Comparison of Non-Invasive Vessel Fractional Flow Reserve Calculated From Angiographic Images Versus Fractional Flow Reserve in Patients With Intermediate Coronary Artery Stenoses; MI: myocardial infarction; NHPR: non-hyperaemic pressure ratio; QFR: quantitative flow ratio; vFFR: vessel fractional flow reserve | |||||
Table 4. Ongoing studies investigating the optimal timing for complete revascularisation in patients presenting with ACS and MVD.
| Study name | Number of patients | Population | Strategy | Comparator | Primary endpoint | Follow-up |
|---|---|---|---|---|---|---|
| STAGED (NCT04918030) | 1,700 | STEMI and MVD | Out-of-hospital staged CR for NCLs(30±15 days) | In-hospital staged CR during the index procedure (7±3 days) | All-cause mortality | 12 months |
| OPTION-STEMI (NCT04626882) | 994 | STEMI and MVD | Immediate FFR-guided CR during primary angioplasty | Staged in-hospital FFR-guided CR for NCLs | Cumulative incidence rate of all-cause death, non-fatal MI, or all unplanned revascularisation | 12 months |
| OPTION-NSTEMI (NCT04968808) | 676 | NSTEMI and MVD | Immediate FFR-guided CR during index PCI | Staged in-hospital FFR-guided CR for NCLs | Cumulative incidence rate of all-cause death, non-fatal MI, or all unplanned revascularisation | 12 months |
| TERMINAL (NCT05231226) | 426 | STEMI and MVD | Immediate CR | Staged CR within 45 days of index pPCI | Composite of all-cause death, ischaemia-driven revascularisation, non-fatal MI and heart failure | 12 months |
| ACS: acute coronary syndrome; CR: complete revascularisation; FFR: fractional flow reserve; MI: myocardial infarction; MVD: multivessel disease; NCL: non-culprit lesion; NSTEMI: non-ST-segment elevation myocardial infarction; OPTION-NSTEMI: OPtimal TIming of Fractional Flow Reserve-Guided Complete RevascularizatiON in Non-ST-Segment Elevation Myocardial Infarction; OPTION-STEMI: OPtimal TIming of Fractional Flow Reserve-Guided Complete RevascularizatiON for Non-Infarct Related Artery in ST-Segment Elevation Myocardial Infarction With Multivessel Disease; pPCI: primary percutaneous coronary intervention; RCT: randomised controlled trial; STAGED: STaged Interventional Strategies for Acute ST-seGment Elevation Myocardial Infarction Patient With Multi-vessel Disease; STEMI: ST-elevation myocardial infarction; TERMINAL: Timing of Complete Revascularization in Patients With ST-segment Elevation Myocardial Infarction And Multivessel Disease-A Multi-center Randomized Controlled Trial | ||||||
Conclusions
Invasive physiological indices of stenosis severity can aid practitioners to optimise management approaches for coronary lesions. While strong evidence supports FFR use during PCI of ACS patients with MVD, further research should address the NSTE-ACS population and the optimal timing for invasive functional-guided PCI of NCLs. Moving forwards, there is significant potential for integrating FFR use into routine care for MVD in patients presenting with ACS, alongside intracoronary imaging and novel physiological indices (Central illustration). Nonetheless, the heterogeneity of this patient cohort means that any strategy should be holistic and individualised to the patient’s needs and preferences.
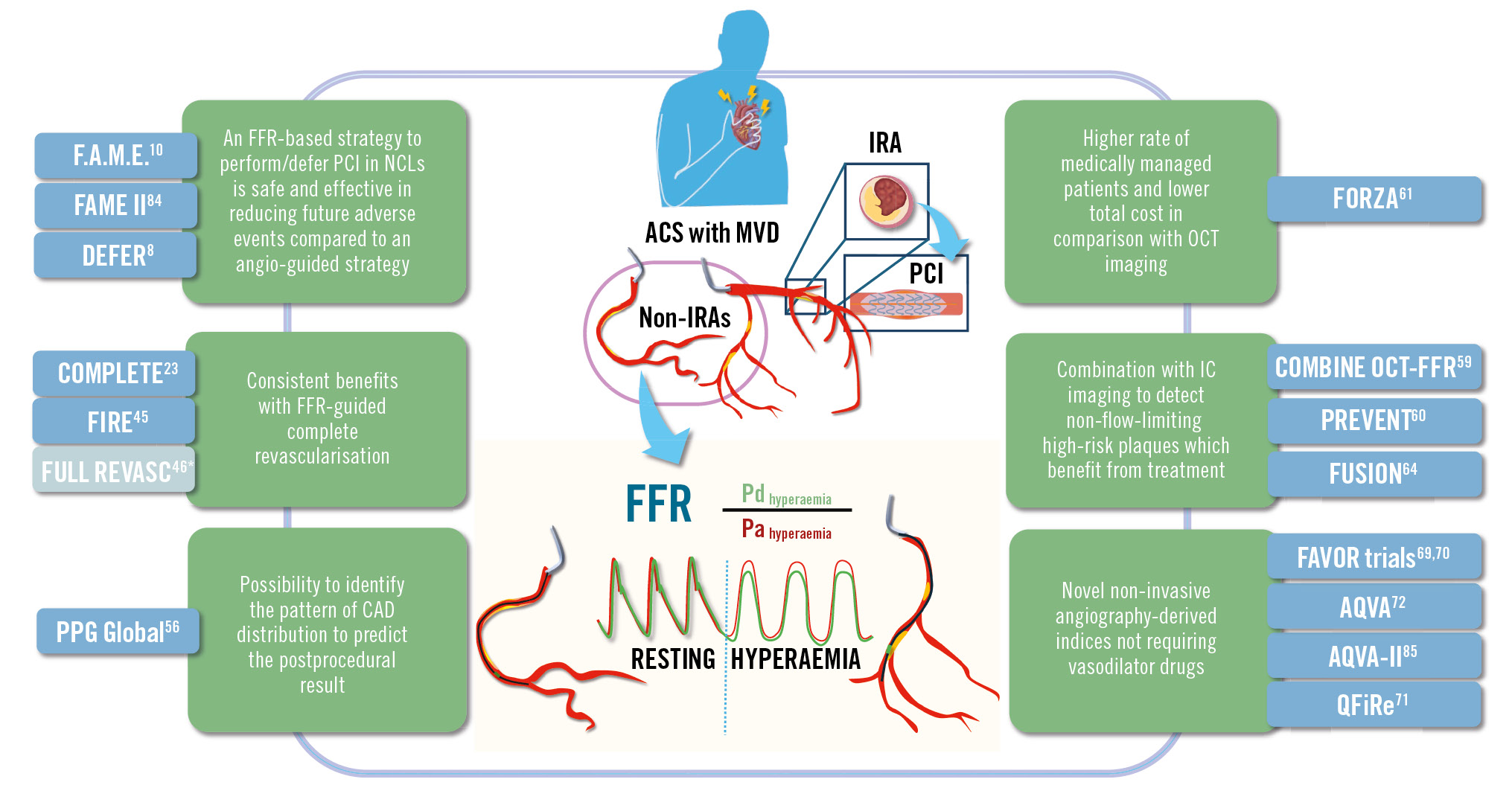
Central illustration. FFR-guided revascularisation in ACS patients with multivessel disease: overview of the evidence supporting its safety and effectiveness in the assessment of non-culprit lesions. *The FULL-REVASC study findings are discordant to the results from other trials that compare physiology-guided complete revascularisation to culprit-only PCI in ACS patients with MVD. ACS: acute coronary syndrome; CAD: coronary artery disease; FFR: fractional flow reserve; IC: intracoronary; IRA: infarct-related artery; MVD: multivessel disease; NCL: non-culprit lesion; OCT: optical coherence tomography; Pa: aortic pressure; PCI: percutaneous coronary intervention; Pd: distal pressure
Conflict of interest statement
The authors have no conflicts of interest to declare.

