Introduction
Stroke during transcatheter aortic valve implantation (TAVI) is not an uncommon complication12, with an occurrence of about 2%3. This often results in significant mortality and morbidity4567. With the extension of TAVI to low-risk patients, stroke prevention takes on increasing importance, as this could adversely affect the physical and cognitive function in a subgroup with a longer life expectancy89. To potentially minimise the risk of strokes, embolic protection devices (EPDs) were designed to help capture embolic debris during TAVI.
The only EPD approved for use by the U.S. Food and Drug Administration to date is the SENTINEL Cerebral Protection System (CPS; Boston Scientific)10. The device comprises two filters, with the proximal filter positioned in the brachiocephalic trunk and the distal filter in the left common carotid artery.
Conflicting data on the efficacy of the SENTINEL device exist in the results of randomised controlled trials (RCTs), although these trials were limited by their small sample sizes111213. A meta-analysis on the subject14 reported a significantly lower risk of stroke, mortality and major bleeding. However, this did not include the recently published PROTECTED TAVR trial, which is the largest RCT to date evaluating the SENTINEL CPS15. The PROTECTED TAVR trial, comprising 3,000 patients, showed no significant reduction in clinical stroke after using the SENTINEL device. However, there was a significant reduction in the secondary endpoint of disabling stroke. This updated meta-analysis, which includes the PROTECTED TAVR trial, aims to evaluate the clinical efficacy and safety of the SENTINEL CPS.
Methods
LITERATURE SEARCH
This meta-analysis was written in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines (Supplementary Appendix 1). A comprehensive literature search using the Cochrane Library and PubMed databases was performed for relevant articles up to September 2022. The following Medical Subject Heading (MeSH) words were used for the search: “Embolic Protection Devices”, “SENTINEL Cerebral Protection System”, “Transcatheter Aortic Valve Implantation”, and “Transcatheter Aortic Valve Replacement”. The bibliographies of relevant studies were also hand-searched to find more studies which met our inclusion criteria. There were no language restrictions applied.
STUDY SELECTION AND OUTCOMES
Publications were reviewed independently by one author (N. Tan) and were included if they reported at least 1 of the following clinical outcomes: all stroke, disabling stroke, non-disabling stroke, mortality, transient ischaemic attack (TIA), acute kidney injury (AKI), major bleeding complications, and major vascular complications. The primary focus was on ischaemic strokes − for which the SENTINEL device is primarily designed to prevent − and thus, haemorrhagic strokes, if reported, were excluded. Some studies, however, did not differentiate between ischaemic and haemorrhagic strokes, and we were unable to exclude the latter in these cases. Other inclusion criteria were studies reporting brain magnetic resonance imaging (MRI) radiological outcomes with respect to ischaemic cerebral lesions post-TAVI. Exclusion criteria included single-arm studies (which only evaluated the efficacy of TAVI with the SENTINEL CPS), studies where other EPDs were included and studies which duplicated databases (for which we included the study with the larger patient pool). This selection process is visually depicted in Supplementary Figure 1.
DATA EXTRACTION AND QUALITY ASSESSMENT
Conflicts regarding the inclusion of studies were discussed and resolved with a second author (J. Yap). The risk of bias was assessed using the Cochrane Collaboration’s tool for assessing risk of bias in randomised controlled trials (RoB 2.0)16 and Risk of Bias in Non-randomized Studies – of Interventions (ROBINS-I) tool17. These results are shown in Supplementary Table 1.
STATISTICAL ANALYSIS
Clinical outcomes were analysed based on timepoints – overall outcomes (up to 30 days) and short-term outcomes (≤7 days). Studies which only provided ≤7-day outcomes were included in the eventual overall outcomes as well. If a study provided both >7-day and ≤7-day outcomes, these were included under both overall and short-term outcomes, respectively. The stroke rates at ≤7 days may portray a more accurate picture of periprocedural outcomes18, as other factors (such as atrial fibrillation) may confound complication rates at the 30-day mark. Thirty-day complication rates help to capture delayed complications for better safety assessment. We utilised the formulae suggested by Wan et al19 to estimate the mean and standard deviation if the study only provided the median and interquartile range for that outcome. We estimated the standardised mean difference (SMD) and odds ratio (OR) via the random-effects model using the Mantel-Haenszel method, with 95% confidence intervals (CI), for radiological and clinical outcomes, respectively. Heterogeneity between studies was evaluated using the Q statistic and I2 test. A sensitivity analysis including only the RCTs was performed for overall outcomes; there were insufficient data to do so for short-term outcomes.
The presence of publication bias was assessed by visually examining the degree of symmetry in Begg’s funnel plot (Supplementary Figure 2) between treatment effects and their standard error (SE) as well as with Egger’s test. Funnel plots were constructed when there were at least 5 studies assessing the association of the SENTINEL device with a particular outcome. A vertical line indicates the estimate based on the model. A pseudo confidence interval region is drawn around this value with bounds equal to ±1.96 times the SE. The studies outside of the pseudo confidence interval region are labelled. Funnel plots may show asymmetry in the absence of publication bias20. To investigate whether heterogeneity may be further explained by differences in characteristics of the studies, we performed univariate regression on age, sex and Society of Thoracic Surgeons (STS) score. Data were extracted and analysed by another independent author (G. Fei) using R with the metafor package (R Foundation for Statistical Computing)21 for meta-analysis.
Results
A total of 15 publications involving 294,134 patients (25,910 with the SENTINEL device and 268,224 without the SENTINEL device) were included. These comprised 4 randomised controlled trials, 8 propensity-matched and 3 cohort studies. The mean age was 80.6 years old, the mean proportion of males was 50.7%, and the mean STS score was 5.27 (Table 1).
Table 1. Summary of included studies.
| Author,year | Typeof study | SENTINELarm (n) | No SENTINEL arm (n) | Outcomes studied | Timing of follow-up | Age (years) | Male (%) | STS score | Type of valve |
|---|---|---|---|---|---|---|---|---|---|
| Haussig et al, 2016# 12 | Randomised controlled trial | 50 | 50 | Mortality, all stroke, disabling stroke, non-disabling stroke, AKI, major vascular complications, major bleeding complicationsMedian total new lesion volume, median number of new lesions | 2 days, 7 days, 30 days*2 days, 7 days for neuroimaging |
80.0±5.1 (SENTINEL)79.3±4.1 (No SENTINEL) | 43.0% (43/100) | 5.6±3.2 (SENTINEL)5.2±2.7 (No SENTINEL) | Medtronic CoreValve |
| Kapadia et al, 201713 | Randomised controlled trial | 234 (121 in device arm, 123 in safety arm) | 119 | Mortality, all stroke, disabling stroke, non-disabling stroke, TIA, AKI, major vascular complicationsMedian total new lesion volume, median number of new lesions | 30 days2-7 days for neuroimaging | 83.4 (78.0-88.2) | 47.9% (174/363) | 6.0 (4.2-8.1) | Medtronic CoreValve/Evolut R, Edwards SAPIEN XT/3 |
| Kapadia et al, 202215 | Randomised controlled trial | 1,501 | 1,499 | Mortality, all stroke, disabling stroke, non-disabling stroke, TIA, AKI, major vascular complications, major bleeding complications | 3 days or in-hospital (whichever earliest) | 78.9±8.0 (SENTINEL)78.9±7.8 (No SENTINEL) | 60.1%(1,803/3,000) | 3.3±2.7 (SENTINEL)3.4±2.8 (No SENTINEL) | Medtronic Evolut R/Evolut PRO, Edwards SAPIEN 3, Lotus, ACURATE, Portico |
| Van Mieghem et al, 201611 | Randomised controlled trial | 32 | 33 | Mortality, all stroke, disabling stroke, non-disabling stroke, AKI, major vascular complications, major bleeding complicationsMedian total new lesion volume, single lesion volume, median number of new lesions | <24 hours, 30 days*, 6 months5 days for neuroimaging |
82 (78-85) | 52.0% (34/65) | 4.8 (3.4-7.2) | Medtronic CoreValve, Edwards SAPIEN XT/3, Portico |
| Alkhouli et al, 2020§ 31 | Propensity-matched | 2,732 | 8,253 | Mortality, all stroke, disabling stroke | In-hospital | 80.0±9.2 (SENTINEL)80.1±9.2 (No SENTINEL) | 58.5% (SENTINEL)55.7% (No SENTINEL) | – | – |
| Butala et al, 202132 | Propensity-matched | 12,409 | 110,777 | Mortality, all stroke, major bleeding complications | 30 days*, in-hospital | 79.0±8.9 (SENTINEL)79.4±8.8 (No SENTINEL) | 55.1% (67,920/123,186) | – | Medtronic CoreValve, Edwards SAPIEN |
| Isogai et al, 202237 | Propensity-matched | 1,802 | 1,037 | Mortality, all stroke, TIA | 3 days or in-hospital (whichever earliest) | 79.2±9.5 | 58.5% | 4.5 (2.9-7.0) (SENTINEL)6.0 (4.2-8.6) (No SENTINEL) | Medtronic CoreValve/Evolut R/Evolut PRO/Evolut PRO+, Edwards SAPIEN XT/3/3 Ultra |
| Kroon et al, 201933 | Propensity-matched | 333 | 333 | Mortality, all stroke, disabling stroke, non-disabling stroke, TIA, AKI, major vascular complications, major bleeding complications | Procedural, 24 hours, 30 days*, in-hospital |
81 (76-85) | 49.2% (328/666) | 4.4 (3.0-6.6) | Medtronic CoreValve/Evolut R, Edwards SAPIEN XT/3, Lotus, Portico |
| Khan et al, 2021¶ 34 | Propensity-matched | 4,380 | 103,935 | Mortality, all stroke, TIA, AKI, major vascular complications, major bleeding complications | In-hospital | 81 (74-87) (SENTINEL)81 (75-86) (No SENTINEL) | 53.7% (58,160/108,315) | – | – |
| Seeger et al, 201718 | Propensity-matched | 280 | 280 | Mortality, all stroke, disabling stroke, non-disabling stroke, AKI, major vascular complications, major bleeding complications | 7 days | 80.6±6.0 (SENTINEL)80.9±6.4 (No SENTINEL) | 54.6% (306/560) | 6.2±4.2 (SENTINEL)6.9±5.0 (No SENTINEL) | Medtronic Evolut R, Edwards SAPIEN 3, Lotus |
| Seeger et al, 201935 | Propensity-matched | 533 | 533 | All stroke, disabling stroke, non-disabling stroke | 3 days | 81.2±7.1 (SENTINEL)81.0±6.6 (No SENTINEL) | 47.1% (502/1,066) | 6.4±4.2 (SENTINEL)6.6±4.9 (No SENTINEL) | – |
| Stachon et al, 2021## 30 | Propensity-matched | 1,564 | 40,090 | Mortality, all stroke, AKI | In-hospital | 80.6±6.4 (SENTINEL)81.1±6.0 (No SENTINEL) | 46.2% (SENTINEL)46.8% (No SENTINEL) | – | – |
| Dona et al, 2022 ‡36 | Cohort | 213 | 198 | Mortality, all stroke, disabling stroke, non-disabling stroke, TIA, AKI | 3 days*, 12 months |
80.4±6.7 | 52.6% (216/411) | – | Medtronic Evolut R, Portico, Symetis ACURATE, Centera Valve, Allegra Valve NVT |
| Seeger et al, 202038 | Cohort | 92 | 904 | All stroke, disabling stroke | 3 days, 30 days*, in-hospital |
80.8 | 49.2% | 6.0±6.9 | Lotus |
| Voss et al, 201839 | Cohort | 39 | 352 | AKI, major vascular complications | In-hospital | 79.1±7.3 (SENTINEL)79.5±7.2 (No SENTINEL) | 50.9% (199/391) | 3.5±2.1 (SENTINEL)4.4±4.2 (No SENTINEL) | Medtronic CoreValve/Evolut R, Edwards SAPIEN 3 |
| Values are presented as mean±standard deviation or median (interquartile range). #In the CLEAN-TAVI trial by Haussig et al, 2 day and 7-day outcomes were not used, as 3-day outcomes by Seeger et al 2019 were used instead (The latter study pooled patients from CLEAN-TAVI, SENTINEL-Ulm, SENTINEL US IDE trial). Two-day timepoints for neuroimaging were also used over the 7-day timepoints. §In this study, although it was not explicitly stated that all devices used were SENTINEL, it was presumed so, as patients in the device group were selected between 1 July 2017 and 31 December 2018, when the SENTINEL was the only approved EPD in the US market. ¶In this study, it was not explicitly stated that all devices used were SENTINEL, but it was presumed so, as the study was based in the USA where the SENTINEL is the only FDA-approved device. ##In this study, although it was not explicitly stated that all devices used were SENTINEL, but it was presumed that majority was SENTINEL as the SENTINEL received approval in Europe in 2014 and patient data collected was from 2015 to 2017. ‡Only the types of self-expanding valves were elaborated on, which comprised 64.0% (263/411) of the patient pool. There was no mention of balloon-expandable valves. Other notes: *In studies with multiple timepoints, the * indicates the timepoint which was used in our meta-analysis for overall outcomes. For male %, separate values for the SENTINEL and no SENTINEL in % were provided if study did not provide the exact number of patients to calculate an overall average male % between the 2 groups. AKI: acute kidney injury; EPD: embolic protection device; FDA: U.S. Food and Drug Administration; STS: Society of Thoracic Surgeons; TIA: transient ischaemic attack | |||||||||
OVERALL OUTCOMES
Regarding overall outcomes (Figure 1), significant reductions were noted for mortality (OR 0.60, 95% CI: 0.41-0.88; p=0.008), all stroke (OR 0.64, 95% CI: 0.46-0.88; p=0.006) and disabling stroke (OR 0.42, 95% CI: 0.23-0.74; p=0.003) in the SENTINEL group. No significant differences were reported for non-disabling stroke (p=0.46), TIA (p=0.26), AKI (p=0.19), major bleeding complications (p=0.11) or major vascular complications (p=0.41). There was significant heterogeneity across the studies for mortality (I2=69.7%; p=0.013) and all stroke (I2=75.0%; p=0.003). A sensitivity analysis (Supplementary Figure 3) was performed including only the 4 RCTs. There were no differences in mortality (OR 0.95, 95% CI: 0.35-2.61; p=0.92) or all stroke (OR 0.74, 95% CI: 0.50-1.10; p=0.14) when comparing the SENTINEL versus no SENTINEL groups. However, there was a significant reduction in the rate of disabling stroke (OR 0.39, 95% CI: 0.17-0.89; p=0.026) in the SENTINEL group. The visual examination of the funnel plots and the results of Egger’s test suggested no publication bias for overall mortality (p=0.83) and low publication bias for all stroke (p=0.049).
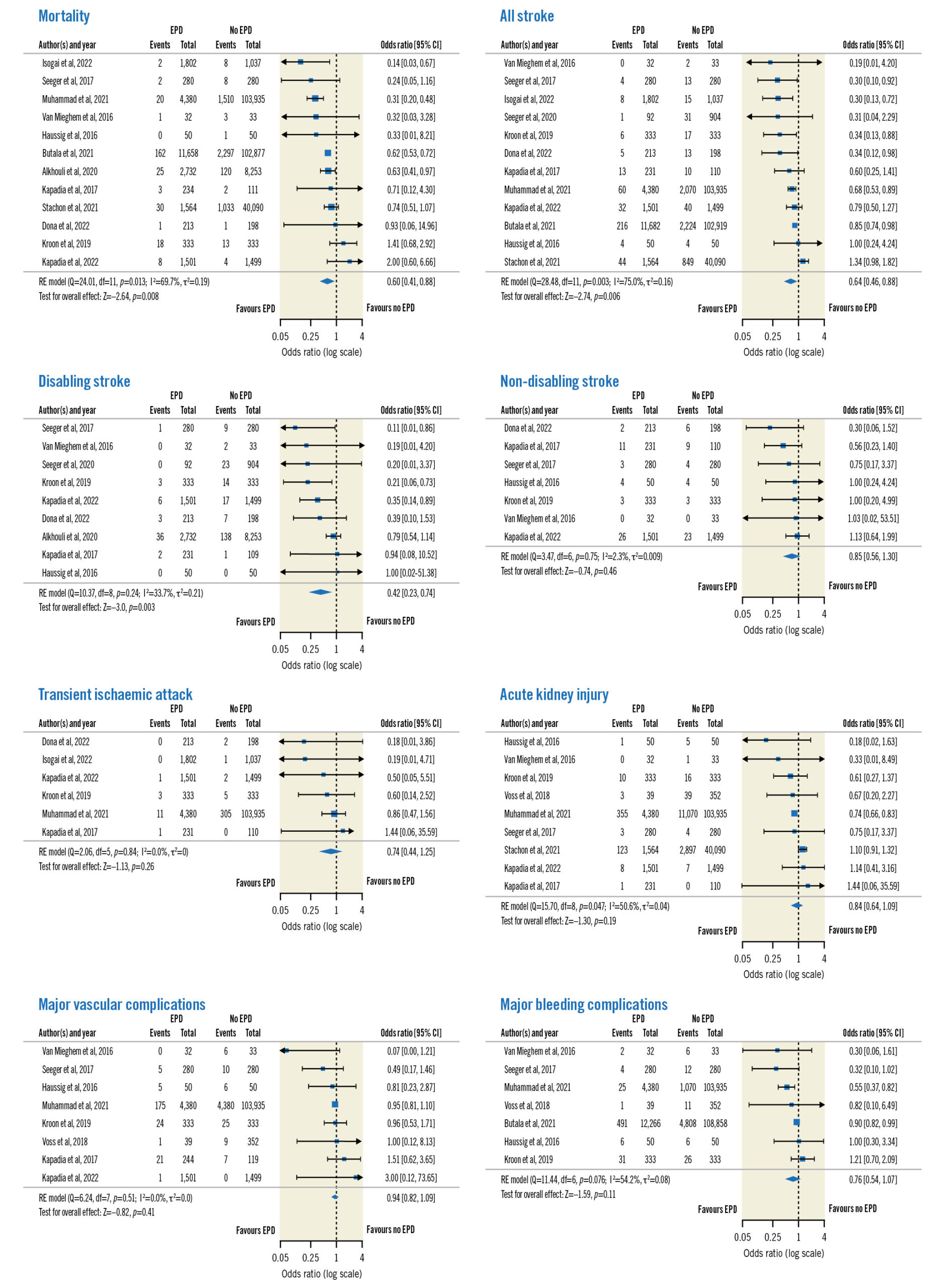
Figure 1. Forest plots of overall outcomes. CI: confidence interval; EPD: embolic protection device; RE: random effects
SHORT-TERM OUTCOMES
For the studies reporting ≤7-day outcomes (n=8) (Figure 2), significant reductions were noted for all stroke (OR 0.42, 95% CI: 0.27-0.65; p<0.001), disabling stroke (OR 0.28, 95% CI: 0.15-0.50; p<0.001) and major bleeding complications (OR 0.29, 95% CI: 0.10-0.79; p=0.02) in the SENTINEL group. No significant differences were reported for mortality (p=0.50), non-disabling stroke (p=0.20), major vascular complications (p=0.46) or AKI (p=1). There was no significant heterogeneity across all the outcomes (p>0.05).
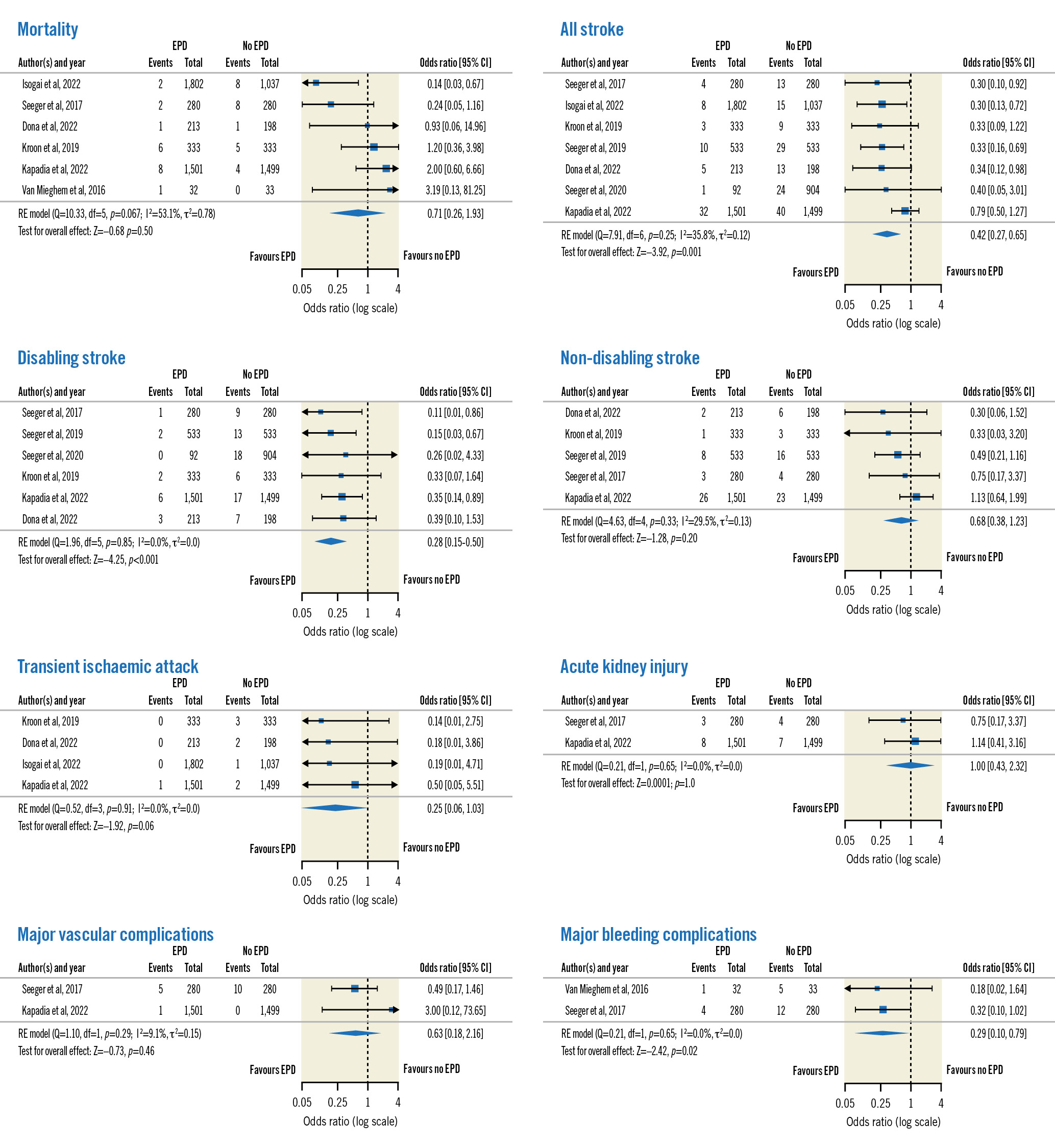
Figure 2. Forest plots of short-term outcomes. CI: confidence interval; EPD: embolic protection device; RE: random effects
NEUROIMAGING OUTCOMES
For neuroimaging outcomes (n=3) (Figure 3), no significant differences in total new lesion volume (SMD −0.48, 95% CI: −1.00 to 0.04; p=0.07), number of new lesions (SMD −0.52, 95% CI: −1.35 to 0.31; p=0.22) or patients with new lesions (OR 0.54, 95% CI: 0.12-2.40; p=0.42) were seen in the SENTINEL group compared to the control group. Significant heterogeneity was noted for total new lesion volume (I2=76.3%; p=0.006) and number of new lesions (I2=90.4%; p=0.001).
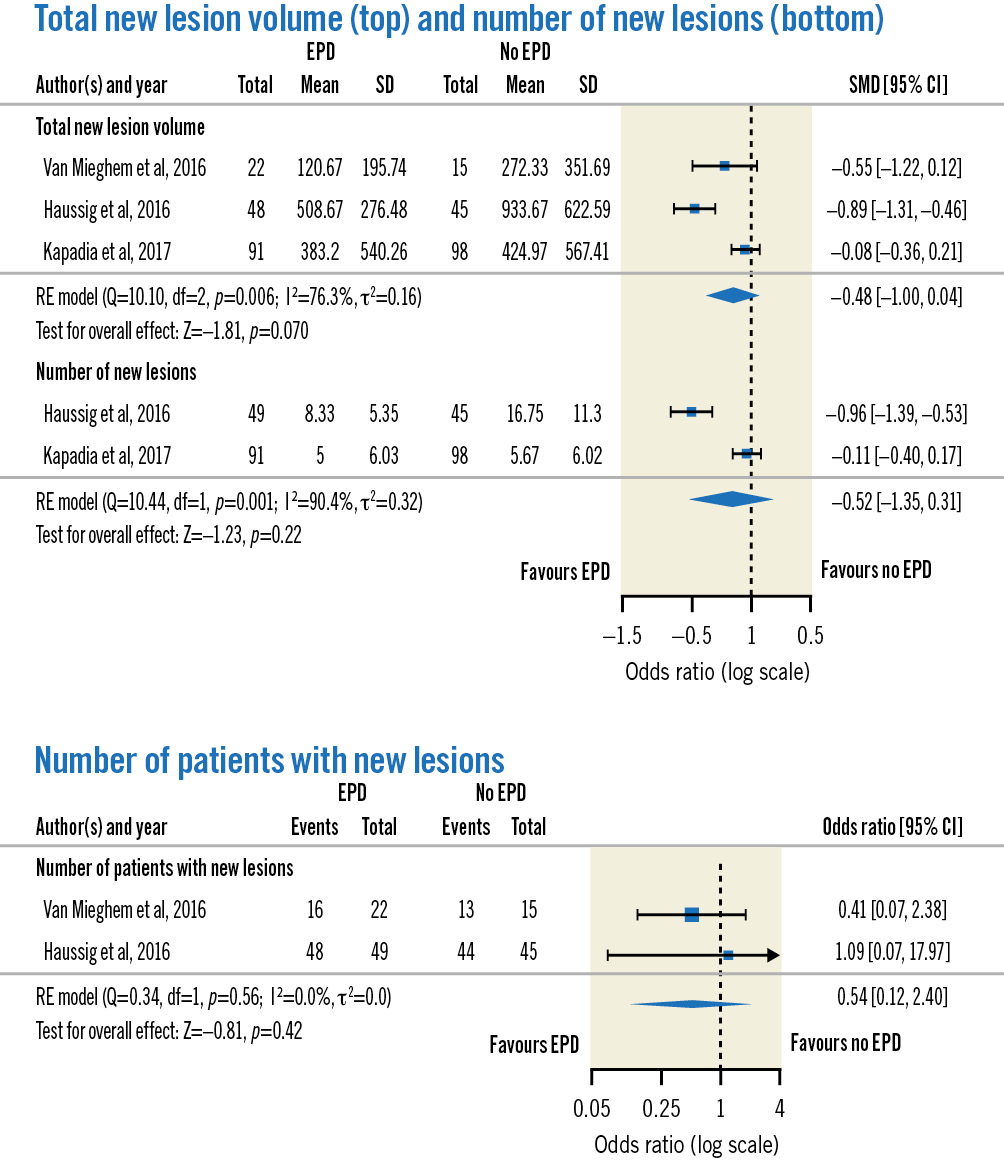
Figure 3. Forest plots of neuroimaging outcomes. CI: confidence interval; EPD: embolic protection device; RE: random effects; SD: standard deviation; SMD: standardised mean difference
Discussion
In this large, updated meta-analysis including the recently published PROTECTED TAVR trial, the important findings are as follows: 1) Cerebral embolic protection with the SENTINEL device was associated with significantly lower rates of mortality, overall stroke and disabling stroke, with short-term (≤7-day) data showing a similar reduction in overall and disabling stroke and randomised data primarily showing a reduction in disabling stroke. 2) There were no significant differences in neuroimaging outcomes (albeit these data were only available in a smaller number of studies). 3) There were no significant differences in TIA, AKI, major bleeding complications or major vascular complications.
EFFICACY
Strokes tend to occur periprocedurally72223, with 50% occurring within 2 days. In this study, significant reductions in the incidence of all stroke and disabling stroke were noted. This reduction in stroke rate has been corroborated in several meta-analyses on the topic. Radwan et al24 showed a reduction in stroke (OR 0.82, 95% CI: 0.71-0.94; p=0.004) with use of the SENTINEL device. Similarly, Ndunda et al14 showed significant reductions in all stroke (OR 0.51, 95% CI: 0.29-0.90; p=0.02) at 30 days. This may potentially be attributed to the mechanism of the device itself, which involves capturing embolic debris during the procedure and, subsequently, lowering the rates of ischaemic stroke within the periprocedural period. Including only randomised data, there was a reduction in the rate of disabling stroke but not for all stroke. The PROTECTED TAVR trial15 randomised 3,000 patients and showed no significant difference in the occurence of clinical stroke between the SENTINEL and the control groups (2.3% vs 2.9%; p=0.30). While the trial was not powered to evaluate disabling stroke, results showed that it occurred in significantly fewer patients in the SENTINEL group compared to the control group (0.5% vs 1.3%; p=0.02).
In addition, we observed a significant reduction in mortality; this was also reported by both Radwan et al24 and Ndunda et al14. However, numerous studies have noted a significant increase in mortality and morbidity in patients with stroke post-TAVI62526. This reduction in mortality with the device may, in part, be from a reduction in the stroke rate, especially disabling strokes. Of note, when only randomised data were included, this impact on mortality was attenuated. The PROTECTED TAVR trial did not show any difference in mortality between the cerebral embolic protection (CEP) and the control groups (0.5% vs 0.3%). Whether the increased sample size and power in meta-analysis are required to show such a mortality effect, or if the confounders not addressed in non-randomised data led to such results will be the work for future larger-scale randomised trials to address.
Neuroimaging may provide additional information, as new subclinical ischaemic lesions after TAVI are seen in approximately 60% to 90% of patients on a brain MRI1227. New lesions are often seen in both hemispheres and are multiple8 – further suggestive of an embolic source. There is a discrepancy between clinically apparent strokes and the incidence of new ischaemic lesions seen on MRI post-TAVI, suggesting that some lesions might be silent. These lesions may affect long-term cognitive status, as studies have shown that silent lesions may cause a more pronounced cognitive decline and an increased risk of dementia2829. This meta-analysis did not show a significant reduction in total new lesion volume, number of new lesions or patients with new lesions on neuroimaging. The reasons for these findings could be multifactorial, as cerebral embolisation during TAVI is still possible even with the SENTINEL device. First, the SENTINEL CPS only has filters covering the brachiocephalic trunk and left common carotid artery, thus, leaving the left vertebral artery unprotected and susceptible to embolism. Second, manipulation of the SENTINEL device within atherosclerotic arteries may cause embolism directly. Thrombi may also form on the surface of the protection device itself during the earlier phases of the procedure when full anticoagulation may not yet be in effect30. Third, the SENTINEL filter only has 1 size, and malapposition may occur, leading to incomplete sealing of arteries and allowing embolic debris to slip by. Fourth, embolic debris may be smaller than the pore size of the filters (140 μm in diameter), thus, it can pass through the filter. Lastly, the studies included in this meta-analysis also had several issues including incomplete MRI follow-ups and small numbers.
SAFETY
Use of the SENTINEL system was shown to be safe, with no significant differences in safety outcomes such as AKI, or major vascular or bleeding complications. The safety profile of the device has been corroborated by other meta-analyses, with both Radwan et al24 and Ndunda et al14 showing no significant differences for AKI or major vascular complications at 30 days. The latest PROTECTED TAVR trial15 also showed no major differences between the SENTINEL and the control groups in terms of AKI (0.5% vs 0.5%), and only 1 (0.1%) patient suffered from a vascular complication due to the insertion of the SENTINEL device.
FUTURE DIRECTIONS
The ongoing British Heart Foundation (BHF) PROTECT-TAVI, which is a randomised trial aiming to recruit 7,730 patients, will be assessing the clinical benefits of the SENTINEL device, not only at short-term (72 hours) intervals, but also at longer (12 months) intervals as well. This will provide further and larger-scale data on the safety and efficacy of cerebral embolic protection devices. Beyond randomised clinical trials on the topic, another area of clinical interest is how to identify patients who may benefit from CEP. Thus far, data on this have been mixed, and there is no established scoring system which is routinely deployed in clinical practice to aid in the identification of such patients. This will be the work of future research.
Limitations
There are several limitations of this study. First, the impact of using different types of valves (e.g., balloon-expandable vs self-expanding valves) and procedural techniques (e.g., predilation of the aortic valve, transfemoral approach vs alternative approaches) on outcomes was unable to be accounted for. Second, there was variation in the timing of outcomes assessed across the studies. Third, the numbers, as well as sample sizes, for studies which looked at neuroimaging outcomes were small, which rendered them underpowered. Fourth, longer-term cognitive outcomes were not available, and these may be important, as silent infarcts have been linked with neurocognitive decline2829. Lastly, the lack of patient-level data precluded the identification of certain patient subsets who may benefit from CEP. This would be best elucidated with a patient-level data meta-analysis.
Conclusions
In this updated meta-analysis, use of the SENTINEL system was associated with lower rates of mortality, all stroke and disabling stroke, although significant heterogeneity was noted for the studies reporting mortality and all stroke. Including exclusively randomised data, there was only a significant reduction in disabling stroke. No significant adverse outcomes with device use were observed.
Impact on daily practice
Stroke during TAVI is not uncommon, thus, emphasising the importance of its prevention. Our paper, which evaluates the clinical efficacy and safety of the SENTINEL cerebral protection system during TAVI, found that it was associated with lower mortality, all stroke and disabling stroke. We hope these data will provide guidance to TAVI operators on the use of the SENTINEL system.
Conflict of interest statement
J. Yap received speaker honoraria from Biosensors, Biotronik, Boston Scientific, Edwards Lifesciences, Johnson & Johnson, Kaneka, Medtronic, and Terumo. K.W. Ho received speaker fees from Edwards Lifesciences, Medtronic, and Abbott Medical. S.H. Ewe received speaker fees from Abbott Medical, Philips, and GE HealthCare. The other authors have no conflicts of interest to declare.
