Introduction
Severe mitral regurgitation (MR) occurs in up to one quarter of all patients with heart failure and reduced ejection fraction (EF)1 and is associated with a mortality rate of 40 to 50% at 3 years23. While primary progressive MR is caused by a leaflet abnormality or any disturbance of the supporting mitral valve apparatus, secondary/functional MR occurs when there is distortion of the supporting apparatus due to maladaptive left ventricular (LV) remodelling (owing to an ischaemic or dilated cardiomyopathy) or due to left atrial dilatation (often in the presence of chronic atrial fibrillation). Regardless of aetiology, the volume overload of chronic MR results in progressive cardiac remodelling with an increase in LV end-diastolic and end-systolic volumes (LVEDV/ESV), which in turn has a deleterious impact on LV myocardial mechanics.
The optimal management of severe MR in patients with LV dysfunction and prohibitive surgical risk remains uncertain4. Transcatheter mitral valve edge-to-edge repair (TEER) with a MitraClip device (Abbott) is now a well-established treatment option. To date, transapical transcatheter mitral valve replacement (TMVR) using the Tendyne device (Abbott) has been performed in more than 800 patients worldwide and has emerged as a potential therapeutic alternative to conventional mitral valve (MV) surgery for selected patients with MR: 1) those deemed to be at prohibitively high surgical risk and 2) those with LV dysfunction whose complex anatomy means that the likelihood of achieving grade 0 or 1 MR with repair is very low.
The results from 2 large, randomised controlled trials of TEER in the setting of secondary/functional MR were conflicting56. The COAPT Trial showed reduced hospitalisation rates for heart failure and lower all-cause mortality in patients with severe symptomatic MR treated with TEER and optimal guideline-directed medical therapy (GDMT) when compared to GDMT alone. However, results from the MITRA-FR trial (in which patients had less severe MR and higher LV end-diastolic dimensions and indexed volumes) suggest that patients did not have more severe end-stage MR, but rather, more advanced LV dysfunction and, therefore, did not benefit from intervention78. More recently, outcomes of 746 patients undergoing screening for TMVR were reported by the CHOICE-MI registry9. TMVR with 1 of 10 dedicated devices was evaluated against TMVR-ineligible patients referred for either bailout-TEER, high-risk surgery or medical therapy9. TMVR resulted in more predictable MR elimination and sustained functional improvement at 1 year than those undergoing TEER (TMVR: 95.2% and 39.2% vs TEER: 37.2% and 28.8% for <1+MR and all-cause mortality/rehospitalisation for heart failure [HFH], respectively)9.
A direct comparison of clinical outcomes following TEER and TMVR in patients with pre-existing LV dysfunction has not previously been reported. The objective of this study was to compare 30-day and 1-year rates of all-cause and cardiovascular mortality as well as HFH in a real-world cohort of patients with pre-existing LV dysfunction undergoing either TEER or TMVR (Central illustration).
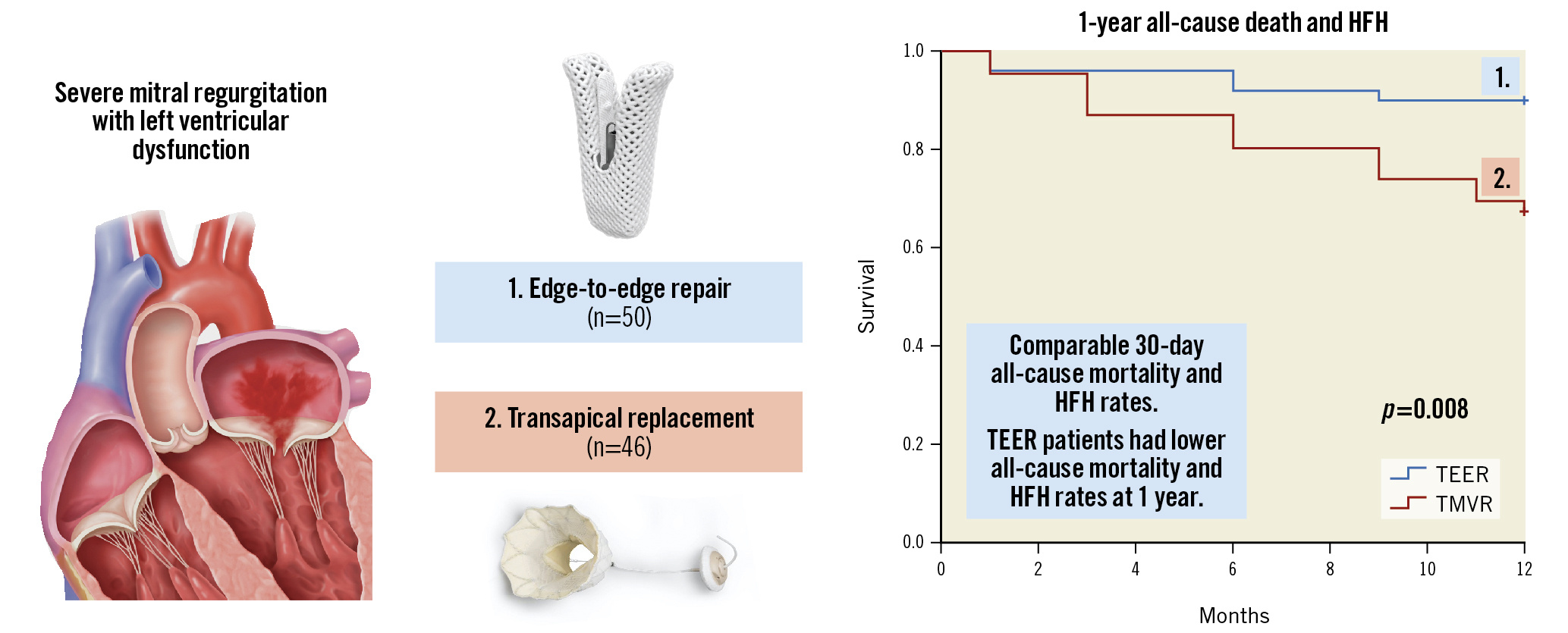
Central illustration. Thirty-day and 1-year outcomes following transcatheter mitral valve edge-to-edge repair versus transapical mitral valve replacement in patients with pre-existing left ventricular dysfunction. Centre top: TEER with the MitraClip device (Abbott). Centre below: TMVR with the Tendyne device (Abbott). Right: Kaplan-Meier analysis showing a higher likelihood of the composite of all-cause death or HF hospitalisation in patients who underwent TMVR. Left: Graphical representation of severe secondary/functional mitral regurgitation from left ventricular dysfunction with failure of leaflet coaptation. Images courtesy of Abbott Vascular, Santa Rosa, CA. HFH: rehospitalisation for heart failure; TEER: transcatheter edge-to-edge repair; TMVR: transcatheter transapical mitral valve replacement
Methods
Study population
Patients with severe MR and LV dysfunction (LVEF ≤50%) who had undergone either TEER or TMVR were identified. The TEER cohort included 50 patients who had undergone edge-to-edge repair using MitraClip at St Vincent’s Hospital prior to December 2019. TEER treatment allocation was decided by a multidisciplinary Heart Team in patients with prohibitive surgical risk and performed by a single experienced operating team (D. Muller/P. Jansz) using 1 or more clips. Details of this technique have been described previously10. The TMVR cohort included 41 patients enrolled in the open-label, non-randomised Tendyne Expanded Feasibility Study (EFS)1112 and 5 patients treated under a “compassionate use” protocol13. TMVR treatment allocation was decided by the local hospital multidisciplinary Heart Team in patients with prohibitive surgical risk but whose complex mitral anatomy was not suitable for TEER. TMVR was performed at 1 of 3 quaternary cardiac centres between 2014 and 2019. Details of the TMVR procedure and EFS patient outcomes at 30 days and 1 and 2 years have been described previously111214.
The following were inclusion criteria for the study: MR grade ≥3+ (primary/mixed or secondary/functional); symptoms of dyspnoea (New York Heart Association [NYHA] Functional Class ≥II); LVEF ≤50% despite the presence of MR; two-dimensional (2D) transthoracic echocardiogram (TTE) prior to treatment, 2D TTE predischarge and postoperatively at 3 months during follow-up; and clinical data available at 30 days and 1 year. Patients with an LVEF >50%, those who were missing clinical or TTE data, or who had undergone conventional MV surgery were excluded. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki with prior approval by each institution’s Human Research Ethics Committee. All demographic and clinical data were manually extracted from the electronic medical records.
Data management and study endpoints
All patients were followed up at 30 days and 1 year post-intervention. The endpoints were 1) the composite of all-cause death and HFH, 2) cardiovascular death and 3) HFH. Death was considered cardiovascular if caused by HF, sudden death, acute myocardial infarction, stroke, procedural or other cardiovascular causes (i.e., rupture of an aneurysm, peripheral ischaemia, or aortic dissection). Patient deaths were identified from hospital records or by contacting patient relatives. Data were verified by the EFS database. As additional endpoints, we reported 1) technical procedural success and 2) reduction in MR severity grades. Procedural success was defined according to the Mitral Valve Academic Research Consortium (MVARC) criteria15. Valve deterioration was defined as significant haemodynamic dysfunction (mean gradient ≥6 mmHg, or MR ≥2+) in the presence of morphological deterioration (e.g., a torn or flail leaflet, calcification, frame fracture, tether rupture or apical pad deterioration). Follow-up was not performed beyond 1 year after device implantation.
Statistical analysis
Categorial variables are reported as number and percentage of observed data. Continuous data are reported as mean±standard deviation (SD) or median (interquartile range [IQR]). All-cause death, cardiovascular death and HFH are reported using Kaplan-Meier estimates. Comparisons between baseline and follow-up parameters were made using the paired Student’s t-test or the Wilcoxon signed-rank test for continuous variables. A 2-tailed probability <0.05 was considered statistically significant. Data analysis was performed with SPSS, version 24 (IBM).
Results
Patient population
Patient characteristics are reported in Table 1 and have been described previously16. Briefly, patients undergoing TEER were older (80±9 vs 72±9 years; p=0.01), but otherwise were well matched with no significant differences in body surface area (1.8±0.2 m² vs 1.9±0.2 m²; p=0.07), estimated glomerular filtration rate (eGFR; 56±20 mL/min vs 53±20 mL/min; p=0.57), serum creatinine (118±108 vs 130±59; p=0.07), resting heart rate (76±20 vs 74±13; p=0.71), or functional score. The Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score was 7±6 in TEER patients and 11±8 in TMVR patients (p=0.42). Twenty-four (48%) TEER patients and 33 (72%) TMVR patients had a history of pre-existing coronary artery disease. Maximal guideline-directed medical therapy was not required for the study. Diuretic prescription was common, however, with 37 (74%) of TEER patients and 41 (89%) of TMVR patients prescribed loop diuretics, and 18 (36%) of the TEER patients and 26 (57%) of the TMVR patients prescribed a mineralocorticoid antagonist (Table 1). Post-procedurally, all TMVR patients were treated with either aspirin (81 to 100 mg daily) or clopidogrel (75 mg daily) and were anticoagulated with intravenous heparin followed by warfarin for ≥3 months (or lifelong in the case of AF), with a target international normalised ratio of 2.5 to 3.5 as per the study criteria. In TEER patients, a regimen of aspirin (100 mg daily) and clopidogrel (75 mg daily) for 3 months was used. In patients with AF or other indications for oral anticoagulation, non-vitamin K antagonist oral anticoagulation or warfarin plus adjuvant single antiplatelet therapy was the preferred strategy.
Ninety-two percent of TEER patients and 100% of TMVR patients were in NYHA Class III/IV heart failure. Eighty percent (77/96) had secondary/functional MR, with a greater proportion of secondary/functional MR in the TMVR group. The underlying pathology was primary/degenerative in 15 (30%) and secondary/functional in 35 (70%) of TEER patients, as opposed to 9% and 91%, respectively, in the TMVR cohort (p<0.05). Baseline TTE characteristics of the study populations are shown in Table 1 and have been previously reported by our group16. Preoperative LVEF was comparable between groups (LVEF 41±9% [TEER] vs 40±10% [TMVR]; p=0.41), as was the LVEDV index (81±43 mL/m² vs 88±24 mL/m²; p=0.20) and the LVESV index (51±34 mL/m² vs 54±20 mL/m²; p=0.34). All patients had preoperative moderate-severe (3+) or severe (4+) MR with comparable flow dynamics (Table 1). No difference in right ventricular (RV) function or pulmonary arterial systolic pressure (PASP; 49±19 mmHg vs 49±16 mmHg; p=0.91) (Table 1) was observed at baseline.
Table 1. Patient characteristics.
| TEER [n=50] | TMVR [n=46] | p-value | ||
|---|---|---|---|---|
| Baseline demographics | ||||
| Age (years), mean±SD | 80±9 | 72±9 | 0.01 | |
| BSA (m²), mean±SD | 1.8±0.2 | 1.9±0.2 | 0.07 | |
| Sex | Male, n (%) | 32 (64%) | 34 (74%) | 0.51 |
| Female, n (%) | 18 (36%) | 12 (26%) | ||
| Race | White, n (%) | 46 (92%) | 44 (96%) | 0.66 |
| Creatinine (µmol/L), mean±SD | 118±106 | 130±59 | 0.07 | |
| eGFR (mL/min), mean±SD | 56±20 | 53±20 | 0.57 | |
| Heart rate (bpm), mean±SD | 76±20 | 74±13 | 0.71 | |
| STS-PROM, mean±SD | 7±6 | 11±8 | 0.42 | |
| Cardiovascular history | Atrial fibrillation, n (%) | 35 (70%) | 38 (83%) | 0.07 |
| NYHA Class 3-4, n (%) | 46 (92%) | 46 (100%) | 0.62 | |
| Hypertension, n (%) | 28 (56%) | 32 (70%) | 0.44 | |
| Coronary artery disease, n (%) |
24 (48%) | 33 (72%) | 0.17 | |
| Medications | ACE inhibitors, n (%) | 28 (56%) | 30 (65%) | 0.62 |
| Beta blockers, n (%) | 25 (50%) | 34 (74%) | 0.05 | |
| Digoxin, n (%) | 14 (28%) | 5 (11%) | 0.15 | |
| Diuretics, n (%) | 37 (74%) | 41 (89%) | 0.09 | |
| MRA, n (%) | 18 (36%) | 26 (57%) | 0.01 | |
| ARNI, n (%) | 0 (0%) | 1 (2%) | 0.32 | |
| CRT, n (%) | 4 (8%) | 7 (15%) | 1.0 | |
| MR mechanism | Primary/mixed, n (%) | 15 (30%) | 4 (9%) | <0.05 |
| Secondary/functional, n (%) | 35 (70%) | 42 (91%) | <0.05 | |
| Transthoracic echocardiogram | ||||
| LVEF (%), mean±SD | 41±9 | 40±10 | 0.41 | |
| LVEDVI (mL/m²), mean±SD | 81±43 | 88±24 | 0.20 | |
| LVESVI (mL/m²), mean±SD | 51±34 | 54±20 | 0.34 | |
| Moderate-severe MR, n (%) | 6 (12%) | 10 (22%) | >0.05 | |
| Severe MR, n (%) | 44 (88%) | 36 (78%) | >0.05 | |
| PISA radius (cm), mean±SD | 0.8±0.2 | 0.8±0.3 | 0.69 | |
| RF (%), mean±SD | 58±19 | 62±19 | 0.55 | |
| EROA (cm²), mean±SD | 0.5±0.3 | 0.5±0.4 | 0.75 | |
| RV (mL/beat), mean±SD | 33±16 | 44±24 | 0.25 | |
| VC (cm), mean±SD | 0.6±0.2 | 0.7±0.3 | 0.07 | |
| PASP (mmHg), mean±SD | 49±19 | 49±16 | 0.91 | |
| ACE: angiotensin-converting enzyme; ARNI: angiotensin receptor neprilysin inhibitor; BSA: body surface area (using Mosteller formula); CRT: cardiac resynchronisation therapy; eGFR: estimated glomerular filtration rate; EROA: effective regurgitation orifice area; LV: left ventricular; LVEDVI: left ventricular end-diastolic volume index; LVEF: left ventricular ejection fraction; LVESVI: left ventricular end-systolic volume index; MR: mitral regurgitation; MRA: mineralocorticoid receptor antagonist; MV: mitral valve; NYHA: New York Heart Association; PASP: pulmonary artery systolic pressure; PISA: proximal isovelocity surface area; RF: regurgitant fraction; RV: regurgitant volume; STS-PROM: Society of Thoracic Surgery Predicted Risk of Mortality; TEER: transcatheter edge-to-edge mitral valve repair; TMVR: transapical transcatheter mitral valve replacement; VC: vena contracta width | ||||
Device implantation
All patients in the TMVR cohort were treated with the transapical Tendyne Mitral Valve System. TEER patients were treated with the MitraClip NT (Abbott) device with an average of 1.7±0.7 clips/patient. Device implantation data are reported in Table 2. Mitral prostheses were successfully implanted in 96% of TEER patients and 100% of TMVR patients. Two (4%) TEER patients suffered leaflet tear. One (2.2%) TMVR patient required retensioning of the apical pad for paravalvular leak (PVL) and haemolysis, 1 (2.2%) developed left ventricular outflow tract (LVOT) obstruction requiring stenting of the LVOT. Two (4%) TEER patients developed a pericardial effusion post-device implantation, which was successfully drained percutaneously. At hospital discharge, 46 (92%) of TEER patients had ≤2+MR whilst no TMVR patient had ≥1+MR (Figure 1). Left ventricular volumes were unchanged post-TEER but significantly reduced post-TMVR (88±23 vs 72±23 mL/m²; p=0.001) (Table 3, Table 4). Device-specific events occurred infrequently in the second postoperative period (30 days to 1 year) in both cohorts. One (2.2%) episode of endocarditis occurred between 30 days and 1 year in the TMVR cohort. Otherwise, there were no reported episodes of femoral vascular access complications, structural valve deterioration, embolisation, device migration, or malposition or fracture in either cohort (Table 2).
Table 2. Device implantation.
| Patients with clinical events | TEER [n=50] | TMVR [n=46] | p-value | |
|---|---|---|---|---|
| Bleeding/haemodynamic compromise, n (%) | Major, extensive, life threatening or fatal† | 0 (0%) | 1 (2.2%) | >0.05 |
| Life threatening†† | 2 (4%) | 0 (0%) | ||
| Fatal | 0 (0%) | 0 0%) | ||
| Device-specific adverse events, n (%) | No. of clips per patient | 1.7±0.7 | N/A | >0.05 |
| Leaflet tear‡ | 2 (4%) | N/A | ||
| Structural valve deterioration | 0 (0%) | 0 0%) | ||
| Embolisation | 0 (0%) | 0 0%) | ||
| Endocarditis‡‡ | 0 (0%) | 1 (2.2%) | ||
| Device migration or malposition | 0 (0%) | 0 0%) | ||
| Fracture | 0 (0%) | 0 0%) | ||
| Haemolysisˆ | 0 (0%) | 1 (2.2%) | ||
| Paravalvular leakˆˆ | 0 (0%) | 1 (2.2%) | ||
| Mitral regurgitation, n (%) |
Nil-trivial | 9 (18%) | 41 (89%) | <0.001 |
| Mild | 29 (58%) | 5 (11%) | ||
| Moderate | 8 (16%) | 0 (0%) | ||
| Moderate-severe | 4 (8%) | 0 (0%) | ||
| Severe | 0 (0%) | 0 (0%) | ||
| †Acute LVOT obstruction treated with 22 mm LVOT stent/VPA-ECMO. ††Pericardial effusion managed with temporary pericardial drain insertion. ‡Small leaflet tear managed conservatively in 1 patient and requiring conversion to open surgery in the other. ‡‡Valvular endocarditis managed with intravenous antibiotics not requiring explant. ˆand ˆˆValvular haemolysis due to paravalvular leak managed with apical re-tensioning. LVOT: left ventricular outflow tract; TEER: transcatheter edge-to-edge mitral valve repair; TMVR: transapical transcatheter mitral valve replacement; VPA-ECMO: veno-pulmonary arterial extracorporeal membrane oxygenation | ||||
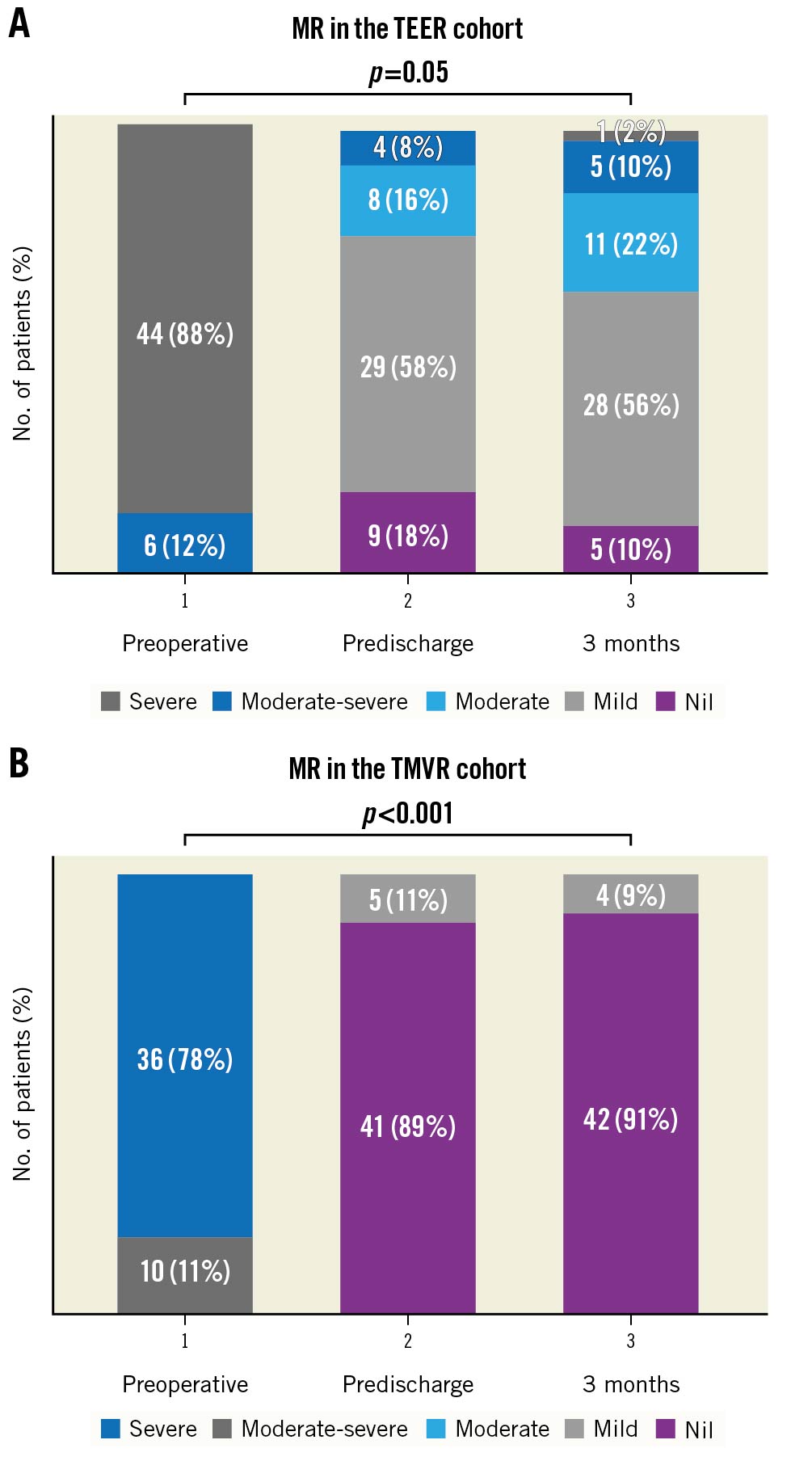
Figure 1. Comparison of postoperative MR. A) Bar chart comparison of pre- and postoperative MR following TEER. All patients had preoperative MR ≥3+. Mitral regurgitation was <2+ in 92% of TEER patients predischarge (p<0.01). At 3 months, 88% of TEER patients still had MR ≤2+. B) Bar chart comparison of pre- and postoperative MR following TMVR. All patients had preoperative MR ≥3+. There was a significant reduction in MR predischarge (p<0.001) and at 3 months (p<0.001). MR: mitral regurgitation; TEER: transcatheter edge-to-edge repair; TMVR: transcatheter transapical mitral valve replacement
Table 3. Comparison of pre-, post- and 3-month TTE characteristics of the TEER study population.
| mean±SD | Pre-(n=50) | Post-discharge (n=50) | 3-months (n=43) | Pre- to post-discharge; Pre- to 3-months | |
|---|---|---|---|---|---|
| LV function | LVEF (%) | 41±11 | 38±10 | 42±12 | p<0.01; p=0.83 |
| LVEDVI (mL/m²) | 81±43 | 81±42 | 81±42 | p=0.52; p=0.52 | |
| LVESVI (mL/m²) | 51±34 | 53±35 | 51±35 | p=0.85; p=0.50 | |
| LVGLS (%) | -13.6±4.1 | -12.6±3.5 | -13.4±4.0 | p=0.14; p=0.91 | |
| LVCS (%) | -15.8±8.4 | -17.4±6.1 | -18.4±6.0 | p=0.64; p=0.62 | |
| LVRS (%) | 11.7±9.5 | 10.2±6.4 | 11.6±8.4 | p=0.66; p=0.52 | |
| RV function | PASP (mmHg) | 49±19 | 43±14 | 41±16 | p=0.03; p<0.01 |
| RVFAC (%) | 28±9 | 31±9 | 32±7 | p=0.07; p=0.10 | |
| RVFWLS (%) | -14.8±7.0 | -16.7±5.2 | -15.8±6.4 | p=0.12; p=0.21 | |
| RVS’ (cm/s) | 7.2±2.5 | 7.8±3.0 | 8.1±2.4 | p=0.04; p<0.01 | |
| TAPSE (cm) | 1.5±0.5 | 1.6±1.5 | 1.6±0.4 | p=0.93; p=0.08 | |
| LV: left ventricular; LVCS: LV circumferential strain; LVEDVI: LV end-diastolic volume index; LVEF: LV ejection fraction; LVESVI: LV end-systolic volume index; LVGLS: LV global longitudinal strain; LVRS: LV radial strain; PASP: pulmonary artery systolic pressure; RV: right ventricular; RVFAC: RV fractional area change; RVFWLS: RV free wall longitudinal strain; RVS’: RV systolic velocity; TAPSE: tricuspid annular plane systolic excursion TEER: transcatheter edge-to-edge mitral valve repair; TTE: transthoracic echocardiogram | |||||
Table 4. Comparison of pre-, post- and 3-month TTE characteristics of the TMVR study population.
| Pre- | Post-discharge (n=46) | 3-months (n=46) | Pre- to post-discharge; Pre- to 3-months | ||
|---|---|---|---|---|---|
| LV function | LVEF (%) | 40±10 | 35±12 | 36±15 | p=0.001; p=0.008 |
| LVEDVI (mL/m²) | 88±24 | 72±23 | 70±40 | p=0.001; p=0.003 | |
| LVESVI (mL/m²) | 54±20 | 45±17 | 48±35 | p=0.033; p=0.328 | |
| LVGLS (%) | -9.6±3 | -9.4±4.4 | -8.2±4.7 | p=0.446; p=0.059 | |
| LVCS (%) | -13.4±5.3 | -16.2±8.9 | -14.1±6.2 | p=0.586; p=0.878 | |
| LVRS (%) | 6.5±4.8 | 5.5±8.9 | 6.5±9.2 | p=0.443; p=0.213 | |
| RV function | PASP (mmHg) | 49±16 | 44±17 | 36±12 | p=0.025; p<0.001 |
| RVFAC (%) | 28±7 | 33±8 | 35±9 | p<0.001; p<0.001 | |
| RVFWLS (%) | -14.2±5.0 | -17.8±6.4 | -17.6±7.3 | p=0.047; p=0.162 | |
| RVS’ (cm/s) | 7.4±2.6 | 7.3±2.1 | 8.2±2.8 | p=0.429; p=0.067 | |
| TAPSE (cm) | 1.0±0.3 | 1.3±0.4 | 1.5±0.5 | p=0.453; p=0.033 | |
| LV: left ventricular; LVCS: LV chamber size; LVEDVI: LV end-diastolic volume index; LVEF: LV ejection fraction; LVESVI: LV end-systolic volume index; LVGLS: LV global longitudinal strain; LVRS: PASP: pulmonary artery systolic pressure; RV: right ventricular; RVFAC: RV fractional area change; RVFWLS: RV free wall longitudinal strain; RVS’: RV systolic velocity; TAPSE: tricuspid annular plane systolic excursion TMVR: transcatheter transapical mitral valve repair; TTE: transthoracic echocardiogram | |||||
30-day clinical outcomes
Clinical outcomes for the 30-day endpoints (first postoperative period) are listed in Table 5. There were no acute intraprocedural deaths in either group. At 30 days, freedom from all-cause death or HF rehospitalisation was 94.8% across the study population. There was no significant difference in freedom from all-cause death or HFH (TEER: n=48 [96%] hazard ratio [HR] 9.3, 95% confidence interval [CI]: 28.34-30.26 vs TMVR: n=43 [93.5%] 95% CI: 27.71-30.12; p=0.585). There was no significant difference in cardiovascular death or HFH (bothp>0.05). During the first 30 days, the causes of death in the TEER cohort were disabling stroke and intractable heart failure. The cause of the single death in the first 30 days in the TMVR cohort was a disabling stroke (Figure 2).
Table 5. Thirty-day and 1-year outcomes.
| 30-day outcomes | TEER [n=50] | TMVR [n=46] | p-value |
|---|---|---|---|
| All-cause death or HF hospitalisation, n (%) | 2 (4%) | 1 (2.2%) | 0.585 |
| CV death or HF hospitalisation, n (%) | 2 (4%) | 1 (2.2%) | 0.585 |
| All-cause death, n (%) | 2 (4%) | 1 (2.2%) | 0.585 |
| CV death, n (%) | 2 (4%) | 1 (2.2%) | 0.585 |
| Rehospitalisation for heart failure, n (%) | 1 (0%) | 0 (0%) | 0.317 |
| 1-year outcomes | TEER [n=50] | TMVR [n=46] | p-value |
| All-cause death or HF hospitalisation, n (%) | 5 (10%) | 15 (32.6%) | 0.008 |
| CV death or HF hospitalisation, n (%) | 4 (8%) | 14 (30.4%) | 0.009 |
| All-cause death, n (%) | 5 (10%) | 7 (15.2%) | 0.358 |
| CV death, n (%) | 4 (8%) | 7 (15.2%) | 0.277 |
| Rehospitalisation for heart failure, n (%) | 2 (4%) | 10 (21.7%) | 0.009 |
| CV: cardiovascular; HF: heart failure; TEER: transcatheter edge-to-edge mitral valve repair; TMVR: transapical transcatheter mitral valve replacement | |||
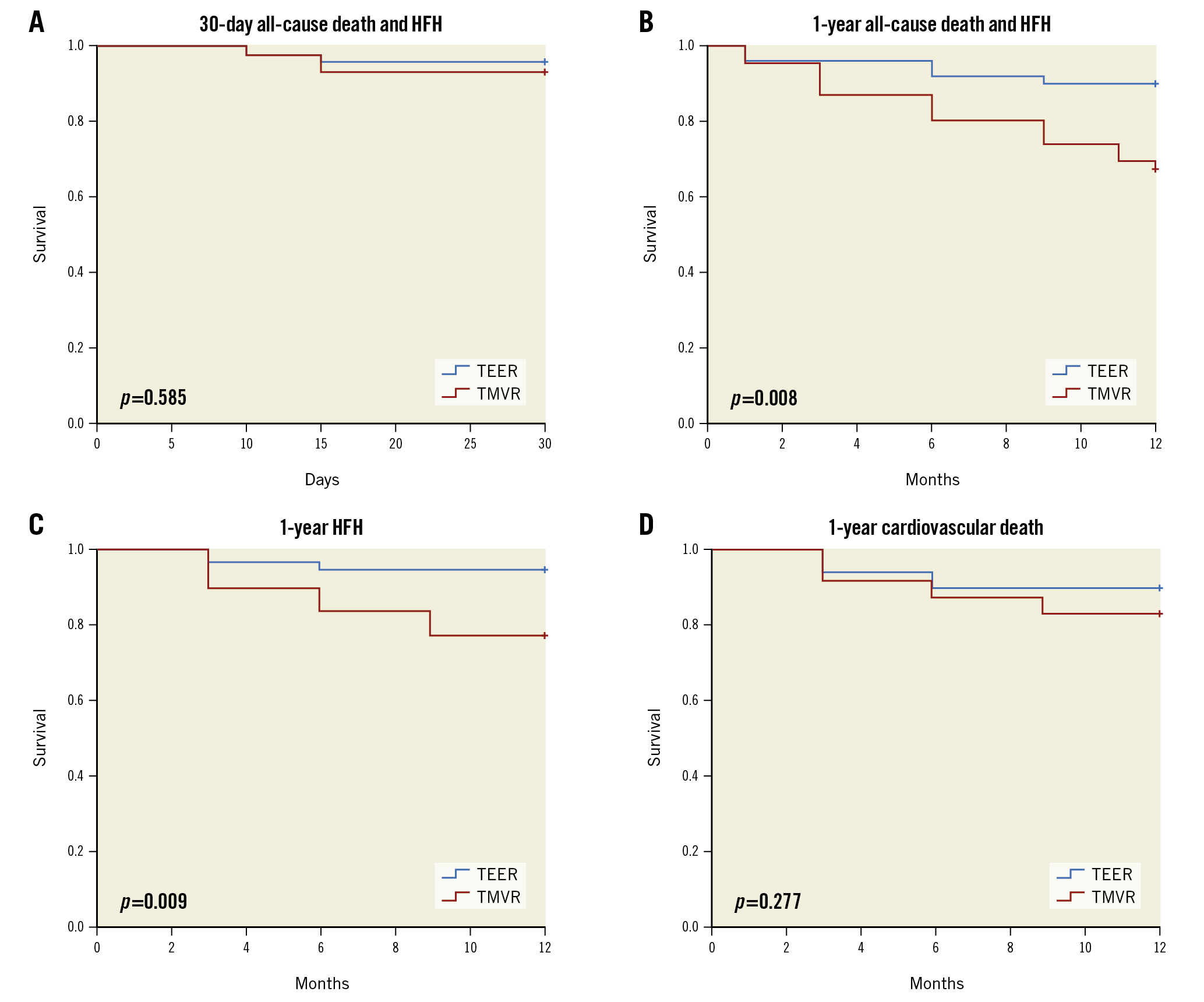
Figure 2. Transcatheter device selection and survival: Kaplan-Meier analysis. No significant differences in clinical endpoints were observed at 30 days (A). In the second postoperative period, Kaplan-Meier analysis showed a higher likelihood of composite of all-cause death or HF hospitalisation in TMVR patients (B). Patients who underwent TMVR had a higher likelihood of readmission for HF (C). However, no significant difference in 1-year cardiovascular death was observed between the 2 groups (D). HF: heart failure; HFH: rehospitalisation for heart failure; TEER: transcatheter edge-to-edge repair; TMVR: transcatheter transapical mitral valve replacement
One-year clinical outcomes
Clinical outcomes for the 1-year endpoints are listed in Table 3. Three (6%) TEER and 6 (13%) TMVR patients died between 30 days and 1 year. The primary endpoint of all-cause death or HF hospitalisation was higher in the TMVR cohort (TEER: n=5 [10%] vs TMVR: n=15 [32.6%]; chi-square p=0.008). At 1 year, freedom from all-cause death or HFH was 79.2% across the entire study population (TEER: 90%, HR 11.26, 95% CI: 10.59-11.93 vs TMVR: 67.4%, 95% CI: 10.09-11.33; p=0.008). For the combined group (n=96), freedom from cardiovascular death was 88.5% (TEER: 92%, HR 11.4, 95% CI: 10.82-11.98 vs TMVR: 84.8%, 95% CI: 10.30-11.75; p=0.277), and rehospitalisation for heart failure was 87.5% (TEER: 96%, HR 11.70, 95% CI: 11.28-12.12 vs TMVR: 78.3%, HR 10.63, 95% CI: 9.81-11.45; p=0.009). In the second postoperative period, the causes of death in the TEER cohort were myocardial infarction and respiratory failure from pneumonia. The causes of mortality in the TMVR cohort after 30 days were cardiac arrest, arrhythmia, heart failure and disabling stroke (Figure 2).
Outcomes stratified by MR classification
A total of 35 TEER and 42 TMVR patients had preoperative secondary MR. Comparable 30-day and 1-year outcomes were observed when stratified by MR type rather than an LVEF <50%. In this subgroup analysis, TEER patients were again older (79±9 vs 73±8 years; p=0.02) but with no significant differences in eGFR, serum creatinine or functional measures, including STS and EuroSCORE II, at baseline (allp>0.05). Left ventricular function (LVEF 38±9 vs 39±19%), size (LVED diameter 62±12 vs 60±6 mm) volume (LVED volume 160±82 vs 165±51 mL) and MR flow haemodynamics (effective regurgitation orifice area [EROA] 0.45±0.31 vs 0.49±0.43 cm²) remained well matched (allp>0.05). At 30 days, freedom from all-cause death or HF rehospitalisation was 98.7% across the study population. There was 1 stroke causing death in the TMVR group, but otherwise, no patients required HFH. At 1 year, freedom from all-cause death or rehospitalisation was 81% across the entire study population (TEER: 93%, HR 12.4, 95% CI: 11.6-13.2 vs TMVR: 74%, HR 11.2, 95% CI: 10.3-12.2; p=0.05). For the combined group (n=77), freedom from cardiovascular death was 90% (TEER: 93%, HR 12.4, 95% CI: 11.6-13.2 vs TMVR: 88%, HR 12.0, 95% CI: 11.1-12.8; p=0.52), and rehospitalisation for heart failure was 89% (TEER: 100%, HR 11.70, 95% CI: 10.4-11.14 vs TMVR: 81%, HR 11.74, 95% CI: 10.71-12.55; p=0.02).
Discussion
The principal findings of this study are (i) TEER and TMVR were both completed safely and effectively in patients with pre-existing LV dysfunction, (ii) TMVR achieved a more complete initial and late reduction in MR than TEER, and (iii) TEER had more favourable 1-year rates of freedom from all-cause death, cardiovascular death, and HF hospitalisation.
Both study cohorts involved patients who were treated during the learning curve of the intervention, using early-generation devices in selected patients with inoperable MR. Despite this, the results achieved with TEER in this study are comparable to large contemporary registries, including COAPT6, the European Registry of Transcatheter Repair for Secondary Mitral Regurgitation (German Clinical Trials Register: DRKS00017428) and the Transcatheter Valve Therapies Register (ClinicalTrials.gov: NCT01737528). It remains unclear whether use of newer-generation devices might have influenced outcomes in the TEER cohort, but the recent introduction of the fourth-generation (XTR and XTW) MitraClip (with longer and wider clips) has clearly improved MR reduction in experienced centres1718.
Correction of mitral regurgitation
Residual MR ≥2+ is widely recognised as an important determinant of increased morbidity and mortality following conventional mitral valve repair surgery192021. MR ≥2+ after TEER has also been associated with increased long-term mortality, and therefore MR ≤2+ has, to date, been considered a surrogate marker of procedural success222324. More recently, Higuchi et al demonstrated that residual MR ≥1+ was associated with increased 2-year mortality (p=0.0031) in a large registry of patients undergoing TEER, highlighting the prognostic importance of residual MR and creating uncertainty as to what might be considered a satisfactory repair outcome25.
One of the most notable findings of this study is that patients undergoing TMVR had more complete and more durable correction of MR as well as an overall reduction in LV volumes. Although 92% of TEER patients achieved MR <2+ predischarge (p<0.01), a procedural success rate comparable with outcomes achieved in the MITRA-FR and COAPT trials56, TMVR eliminated MR altogether predischarge, and these findings were sustained at 1 year. Whether other factors, such as the mechanical effect of the apical tether, may have contributed to the improved LV remodelling after Tendyne TMVR remains to be determined. Despite immediate and sustained elimination of MR in the TMVR cohort, this did not translate to improved clinical outcomes at 1 year as we might have expected.
Transseptal versus transapical approach
There were no intraprocedural deaths in either the TEER or TMVR cohorts, and 30-day mortality was exceedingly low. All-cause death and HFH, however, were significantly higher during the second postoperative period in the TMVR cohort. Although the transapical approach facilitates the placement of large-bore delivery sheaths as well as the coaxial alignment of the prosthesis relative to the native mitral annulus26, the sizing of current delivery sheaths, the relatively thin ventricular myocardium and apical access point have all been associated with ongoing operative mortality in the early TMVR experience (ranging from 6 to 14%) that is, as yet, not fully understood or explained26. Rather, postoperative recovery and 30-day to 1-year mortality using a transapical approach currently more closely mimics that of open surgery rather than other percutaneous mitral valve therapies112728. On the other hand, the transseptal approach used in TEER and selected TMVR device therapies undoubtedly offers an expedited early post-procedural recovery. Whether the transapical approach has late adverse sequelae (i.e., myocardial rupture, arrhythmia) remains unknown and requires further evaluation.
Perioperative heart failure management
In the Expanded Clinical Study of the Tendyne Mitral Valve System (ClinicalTrials.gov: NCT02321514), there were no instances of need for extracorporeal membrane oxygenation, unlike some other TMVR devices; however, 4 patients had intra-aortic balloon pump placement for management of acute LV dysfunction12. A total of 86.7% of patients required intraprocedural inotropic support, although this was discontinued in the early postoperative period for all but one patient12. Currently few dedicated guidelines exist for the perioperative management of high-risk patients undergoing TMVR. This is despite overwhelming evidence that debilitating heart failure, NYHA Functional Class III and IV symptoms, hospitalisation for heart failure within the prior year, and severe comorbidities are frequent. As has been learned from the TEER experience, patients undergoing TMVR should be commenced on optimal guideline-directed medical therapy and managed by a multidisciplinary Heart Team specialising in structural interventions of the mitral valve with particular attention paid to the second postoperative period.
Limitations
There are several limitations of this study. The foremost is the divergent nature of the patient groups: our TEER cohort was older, with a greater proportion of patients with primary/degenerative MR as opposed to the TMVR cohort, which had more patients with pure secondary/functional MR. The study was not large enough to allow propensity-matched analysis, and thus, differences in the 2 populations may confound postoperative outcomes. For example, although the left ventricular size and systolic function was similar between the 2 groups, there may have been important prognostic differences between the groups related to the pathogenesis of the valve disease (secondary versus primary MR) and LV dysfunction (ischaemic versus non-ischaemic) that might account for the difference in clinical outcomes at 1 year. Optimal guideline-directed medical therapy was not an inclusion requirement for either group. Nevertheless, this study represents the first direct comparison of TEER and TMVR in patients with LV dysfunction.
Conclusions
In this study, TVMR was more effective than TEER in correcting MR in patients with pre-existing LV dysfunction, but it was associated with increased rates of all-cause death and HFH in the second postoperative period between 30 days and 1 year.
Impact on daily practice
Transapical TMVR is more effective than TEER in correcting MR in patients with pre-existing LV dysfunction, but it is associated with increased rates of all-cause death and HFH at 1 year.
Acknowledgements
We acknowledge the contribution of St Vincent’s and Royal Brompton Hospital structural heart disease research nurse coordinators Ms Alexis Arrigada, Ms Erika O’Dea and Mr Teijo Palovaara.
Funding
S. Hungerford is supported by an RACP Postgraduate Research Fellowship grant, an Australian Government Department of Education and Research Training Program grant, and a St Vincent’s Clinic Travelling Fellowship grant.
Conflict of interest statement
D.W.M. Muller is an advisory board member and consultant for Medtronic, Edwards Lifesciences, and Abbott Vascular; and has received research grant support from Abbott Vascular and Medtronic.A. Duncan and G. Dahle are consultants for Abbott Vascular. The other authors have no conflicts of interest to declare.

