Introduction
Left main (LM) disease is encountered in at least 4% of patients undergoing coronary angiography1. Mounting evidence supports a percutaneous revascularisation strategy in patients with isolated LM disease or in the presence of overall low-complexity coronary artery disease (CAD)2. When planning a percutaneous coronary intervention (PCI), interventionalists should be aware of the distinctive anatomical features of the left main (Figure 1), including a large diameter (mean 5 mm, range between 3.5 mm and 6 mm)3 and the fact that distal bifurcation is involved in ~80% of the cases4, with a circumflex (CX) artery virtually always being clinically important as it supplies >10% of the myocardium in >95% of patients5. Given the potential challenges associated with percutaneous LM treatment, a thorough understanding of the decision-making process in the daily practice of caring for patients with LM disease is needed. In this article, we review the accumulated evidence in the field of LM revascularisation and attempt to integrate it into a common clinical treatment pathway.
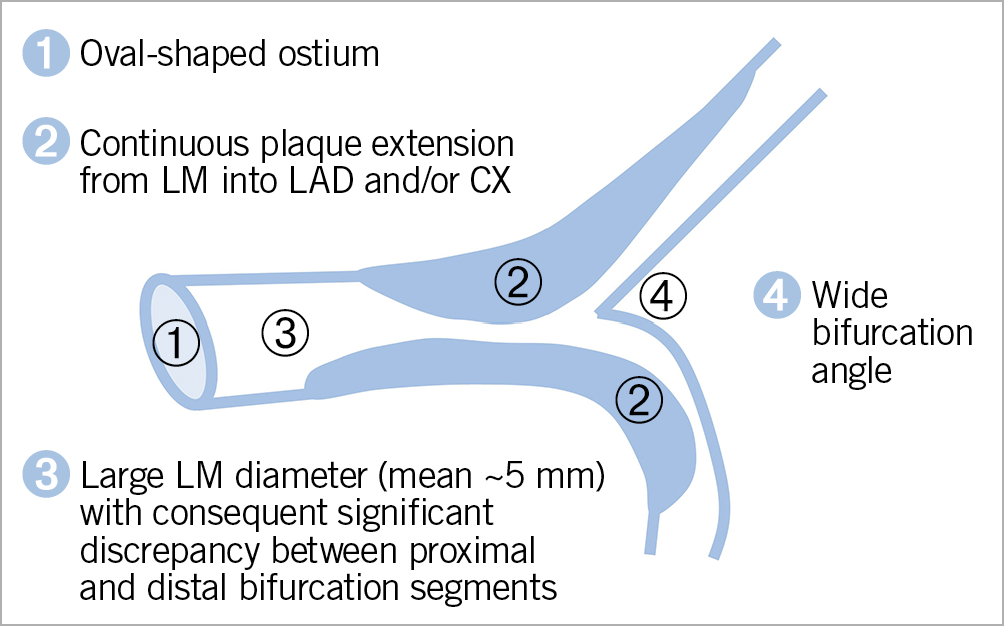
Figure 1. Left main coronary artery anatomy. Distinct anatomical characteristics of the left main, which need to be accounted for when planning percutaneous intervention. CX: circumflex artery; LAD: left anterior descending artery; LM: left main
WHEN TO REVASCULARISE THE LEFT MAIN?
Historically, the primary comparison has been between coronary artery bypass grafting (CABG) and medical therapy, showing a clear survival benefit for revascularisation in patients with LM disease6. A long-term follow-up of 1,484 patients with LM stenosis ≥50% from the CASS registry showed prolonged survival with CABG as compared with medical therapy alone (median 13.3 vs 6.6 years, respectively)7. The recommendation to revascularise patients with angiographic LM stenosis ≥50% persists today2. Modern use of intracoronary imaging and physiology guidance allows for a more detailed assessment of angiographically intermediate lesions in the LM. Revascularisation deferral based on an intravascular ultrasound (IVUS)-measured cut-off of >6 mm2 minimal lumen area (MLA) has been deemed safe, with only 4% of patients requiring subsequent LM revascularisation during two-year follow-up, without myocardial infarctions (MI) reported8. Smaller MLA cut-offs (4.5 and 4.8 mm2) have been proposed in studies enrolling patients of Asian ethnicity9,10. When using intracoronary physiology to assess the haemodynamic relevance of an intermediate LM stenosis, one should bear in mind a possible interaction with downstream disease in the left anterior descending (LAD) and CX arteries11. Whereas a fractional flow reserve (FFR) of >0.80 remains the accepted cut-off for deferring revascularisation in intermediate LM lesions5,12, a recent study confirmed the safety of LM revascularisation deferral based on the instantaneous wave-free ratio (iFR) cut-off of >0.8913. The presented evidence notwithstanding, the decision regarding the revascularisation of an angiographically intermediate LM lesion is a multifactorial one. The morphological plaque features of a haemodynamically insignificant atherosclerotic plaque, such as high lipid content, together with the patient clinical characteristics and the overall extent of coronary artery disease (CAD) should all play a role when deciding whether to revascularise a patient with LM disease. In contemporary practice, an MLA of <4.5 mm2 measured by IVUS is accepted as an indication for LM revascularisation, and MLA >6 mm2 is a threshold for safe deferral and conservative management, whereas an MLA between 4.5 mm2 and 6 mm2 represents a “grey zone” and requires further assessment.
PERCUTANEOUS OR SURGICAL REVASCULARISATION?
Once the decision to revascularise a patient with LM stem disease has been made, the next step is to decide whether a percutaneous or surgical revascularisation strategy is more suitable. The most recent clinical practice guidelines on myocardial revascularisation, issued by the European Society of Cardiology (ESC)2, together with the North American Appropriate Use Criteria for Coronary Revascularization14 recommend considering both anatomical and clinical criteria. Whereas the choice of PCI is supported in patients with isolated LM disease or when paired with low-complexity CAD, a more diffuse and complex coronary anatomy, expressed by a SYNTAX score >22, signals a preference for CABG2,14. From a clinical point of view, PCI is preferred in acute coronary syndromes if the patient is unstable, and more broadly in cases of high periprocedural surgical risk, either based on the Society of Thoracic Surgeons (STS) score, or in the presence of a major co-morbidity not reflected by the score, advanced age, frailty and/or impaired mobility. Diabetes, low left ventricular ejection fraction and contraindications for dual antiplatelet therapy, however, would drive the decision towards CABG2. These recommendations have been largely based on the randomised evidence described in detail in Table 1,4,15,16,17.
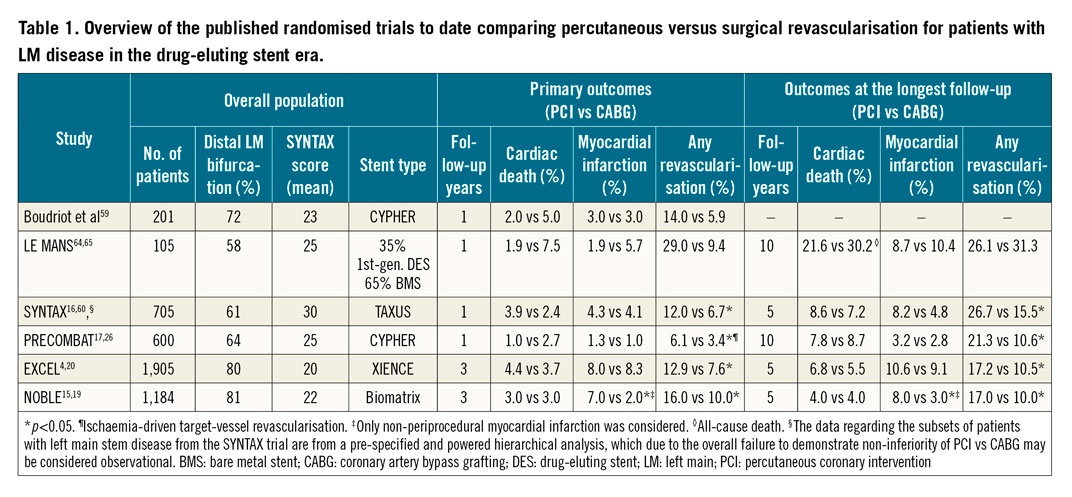
In the era of first-generation drug-eluting stents (DES), two randomised trials, Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery (SYNTAX)16 and Premier of Randomized Comparison of Bypass Surgery versus Angioplasty Using Sirolimus-Eluting Stent in Patients with Left Main Coronary Artery Disease (PRECOMBAT)17, demonstrated the non-inferiority of PCI compared with CABG in terms of the combined primary endpoint of major adverse cardiovascular and cerebrovascular events (MACCE). More recently, the Evaluation of XIENCE versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization (EXCEL)4 trial confirmed the non-inferiority of PCI to CABG for the primary endpoint of death, MI or stroke at three years, whereas in the Nordic-Baltic-British left main revascularisation (NOBLE)15 study, PCI was deemed inferior to CABG. As depicted in Table 1, the overall common direction of the randomised evidence base appears to be that, while no difference in cardiac mortality up to the 10-year follow-up is detectable, there is a clear benefit from CABG, which reduces the need for repeat revascularisation. There remains, however, ambiguity in the rates of new MI. When it comes to interpreting this overall evidence for the purpose of choosing between PCI or CABG in an individual patient, a deeper understanding of the following issues may be needed. First, due to the heterogeneity of the endpoint of repeat revascularisation, the clinical consequences should be carefully considered and discussed with the patient, with the most important question being whether repeat revascularisation increases the risk of mortality. In this respect, a secondary analysis of the EXCEL trial provides a detailed overview of the clinical implications of different modes of repeat revascularisation following PCI or CABG in patients with LM disease18. The main findings were that repeat revascularisation is more frequent after PCI than CABG (12.9% vs 7.6%, at three years, p<0.01) and that its occurrence is associated with increased all-cause (HR=2.05, 95% CI: 1.13-3.70) and cardiovascular mortality (HR=4.22, 95% CI: 2.10-8.48), with most of the risk being confined to the first 30 days after the revascularisation procedure. Furthermore, the risk of cardiac mortality was magnified if repeat revascularisation had to be performed by CABG (HR=10.92), as compared to by PCI (HR=2.88). Importantly, repeating a revascularisation procedure in a previously non-revascularised artery segment (non-target vessel or target vessel but non-target lesion as opposed to target lesion and target vessel revascularisation) was not linked to higher mortality18. The second issue pertains to the definition of myocardial infarction across different trials, more specifically how periprocedural MI (PMI) was defined. This is important, since in the NOBLE trial only non-procedural MI was part of the primary endpoint, and it was reported to be higher in patients undergoing PCI compared with CABG (8.0% vs 3.0%, at five years, p<0.01)19. The EXCEL trial however, which accounted for the extent of periprocedural myocardial injury as part of its primary endpoint, reported no difference in the overall MI rates (10.6% for PCI vs 9.1% for CABG at five years)20. The definition of PMI varies substantially, from an elevation in troponin >5 and >10x upper reference limit (URL), for PCI and CABG respectively in the 4th Universal MI definition21, to >70x the upper reference limit (URL) (or rise in creatine kinase myocardial band [CK-MB] of >10x URL) in the Society for Cardiovascular Angiography and Interventions (SCAI) definition22. In the EXCEL trial, a more conservative definition was adopted (CK-MB rise of >10x URL or >5x URL in case of electrocardiogram [ECG] or imaging findings of ischaemia or angiographic complications), thus preferentially detecting an extensive periprocedural injury that occurs after CABG, while being insensitive to less pronounced periprocedural biomarker elevations that are common after PCI. According to this definition, PMI was more frequent after CABG and it was in fact associated with increased all-cause (HR=2.28, 95% CI: 1.22–4.29) and cardiovascular mortality (HR=2.63, 95% CI: 1.19–5.81) at three years23. Since recent evidence has also shown smaller periprocedural troponin elevations, as defined by the 4th Universal MI definition, to be associated with increased mortality after PCI24, a balanced approach and a thorough patient evaluation is advisable. Finally, the third issue to be considered is the impact of PCI versus CABG on all-cause mortality following treatment for LM disease. Whereas 10-year all-cause mortality did not differ between PCI and CABG in the SYNTAX25 (26.0% vs 28.0%, respectively) and PRECOMBAT26 trials (14.5% vs 13.8%), nor in the in the NOBLE19 study at five years (9% vs 9%), there was a mortality excess associated with PCI after five years in the EXCEL20 trial (13.0% vs 9.9%). A recent pooled analysis of all available randomised trials of PCI versus CABG in patients with LM disease found no difference in all-cause and cardiac mortality, stroke or MI27. In terms of unplanned revascularisation, PCI was inferior to CABG27. Future research may clarify the relative impact of new surgical (e.g., predominant use of arterial grafts) and percutaneous techniques (e.g., increased use of standardised intracoronary imaging guidance) on their respective outcomes.
WHICH LEFT MAIN PCI TECHNIQUE?
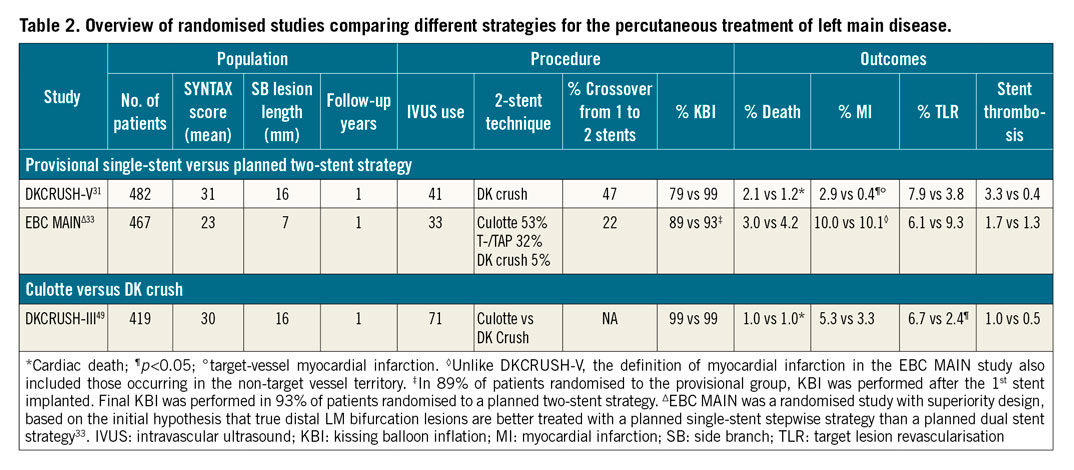
Given the aforementioned results of PCI versus CABG in patients with LM disease, which imply an excess in the rate of repeat revascularisations after PCI (with target lesion revascularisation being most closely associated with mortality)18, the choice of PCI technique may be seen as an effect modifier and a means of improving outcomes following PCI for LM disease. This view is supported by the results of the SYNTAX II study, which demonstrates that new developments in the techniques and technology of PCI are associated with improved clinical outcomes; namely, fewer repeat revascularisations and fewer new MI when compared with a historical cohort of similar patients with three-vessel disease from the SYNTAX I trial28.
ONE VERSUS TWO STENTS
The first decision to be made regarding LM PCI technique is whether a planned single-stent strategy with provisional stenting of the side branch should be preferred over an upfront two-stent technique. In patients without a true distal LM bifurcation lesion, i.e., in cases where the side branch ostium is not significantly affected by disease, provisional stenting is the preferred strategy5,29. A recent secondary analysis from the EXCEL trial reaffirmed this approach, as performing the provisional strategy was associated with a reduction in cardiac mortality as compared with upfront double stenting (6.1% vs 13.0%) in patients without true distal LM bifurcations30. When it comes to patients with true distal bifurcation LM disease, the evidence base consists of two randomised trials. The DKCRUSH-V trial showed the primacy of routine double-kissing (DK) crush stenting in such patients, as compared with the initial provisional strategy. The primary endpoint of one-year target lesion failure (TLF), consisting of cardiac death, target vessel myocardial infarction (TV-MI) and target lesion revascularisation (TLR) was reduced in the DK crush arm (5.0% vs 10.7% in the provisional arm, HR=0.42, 95% CI: 0.21-0.85, p=0.02)31. A follow-up study confirmed this difference in favour of DK crush stenting persisted at three years (8.3% vs 16.9%, p<0.01)32. On the other hand, the similarly designed European Bifurcation Club Left Main Study (EBC MAIN) that compared a stepwise provisional strategy with an upfront two-stent strategy in patients with LM disease affecting both LAD and CX ostia, was neutral in terms of its combined primary endpoint of all-cause death, any MI and TLR at one year (14.7% in the provisional group vs 17.7% in the two-stent group; HR=0.8, 95% CI: 0.5–1.3; p=0.34)33. As seen in more detail in Table 2, several important study characteristics may explain the observed differences in outcomes. First, in terms of anatomical complexity, the DKCRUSH-V trial seems to have enrolled a more complex population of patients with a higher mean SYNTAX score, and, importantly, a longer lesion length in the CX artery (16 mm vs 7 mm in EBC MAIN). Second, the primary endpoint of target lesion failure was tailored to detect differences in stent-related events in the DKCRUSH-V trial, as opposed to the EBC MAIN study which used a more patient-oriented primary endpoint of all-cause death, any MI and TLR. Finally, the patient population in the EBC MAIN study was older (mean age 70 vs 64 in the DKCRUSH-V trial) and more comorbid (e.g., prior stroke 7% vs 1.4%), which could in part explain the higher overall event rates. Overall, when deciding between stepwise provisional and upfront double stenting, such as DK crush, the key determinant remains the complexity of the underlying LM lesion. Even in the DKCRUSH-V trial, the main driver of the benefit attributed to DK crush stenting was the complexity of the distal LM bifurcation lesion, with major criteria being stenosis severity ≥70% and the extension of the disease in the CX ≥10 mm31. This observation was replicated in the recent DEFINITION II trial34, which used the same two major criteria of LM bifurcation lesion complexity. In so defined complex bifurcations, the upfront two-stent strategy reduced the rates of TV-MI and TLR as compared with the provisional approach. Conversely, in patients with a side branch (SB) lesion length <10 mm and lesion severity <70%, there was no significant difference in terms of TLF between the provisional and the DK crush strategy in the DKCRUSH-V trial31. From the perspective of the EBC MAIN study, in which the mean length of the CX disease was also <10 mm and in which a planned two-stent strategy was not superior to a provisional strategy, these results seem to be complementary and relevant for the contemporary decision-making process regarding LM revascularisation strategy.
STEPWISE PROVISIONAL STRATEGY
Given the multitude and heterogeneity of anatomical and clinical features affecting the outcomes of LM PCI, a standardised approach to stenting technique may ensure that established procedural quality markers are met. In this respect, the recently published EBC white paper on stenting techniques may serve as a reference point for predefined procedural steps in provisional single-stent strategy, T- or TAP, culotte and DK crush35 stenting.
The provisional strategy is based on the premise that in most instances of bifurcation PCI, stenting the main branch alone will facilitate a better outcome. Importantly, T- or TAP, and culotte stenting could be used either in the sequence of a stepwise provisional strategy in a bailout indication (dissection, flow compromise, etc.) or as a planned, upfront two-stent strategy. To prevent acute side branch jeopardy and to ensure optimal results in the main vessel the following principles should be adhered to: first, the stent should be sized according to the distal reference diameter, and it should be long enough to accommodate a short post-dilating balloon in the proximal segment35,36. The second mandatory step is post-dilation with a larger-than-stent sized short balloon, positioned with its distal end in front of the carina: the proximal optimisation technique (POT)35,37. Adapting the stent contour to the underlying fractal anatomy of a bifurcation in practical terms means correcting for malapposition and/or underexpansion in the proximal segment (Figure 2),38,39. The difference in the diameters of the proximal versus distal segment of a bifurcation is proportional to the size of the side branch, which explains the crucial role of POT in proximal bifurcations such as LM. In addition, correctly performing POT reduces SB ostium obstruction and facilitates SB rewiring40. Apart from the immediate procedural consequences of not performing POT, such as abluminal side branch rewiring with its associated complications, recent clinical evidence suggests the benefit of POT in reducing the rate of TLF in a large all-comer registry of patients undergoing bifurcation PCI41.
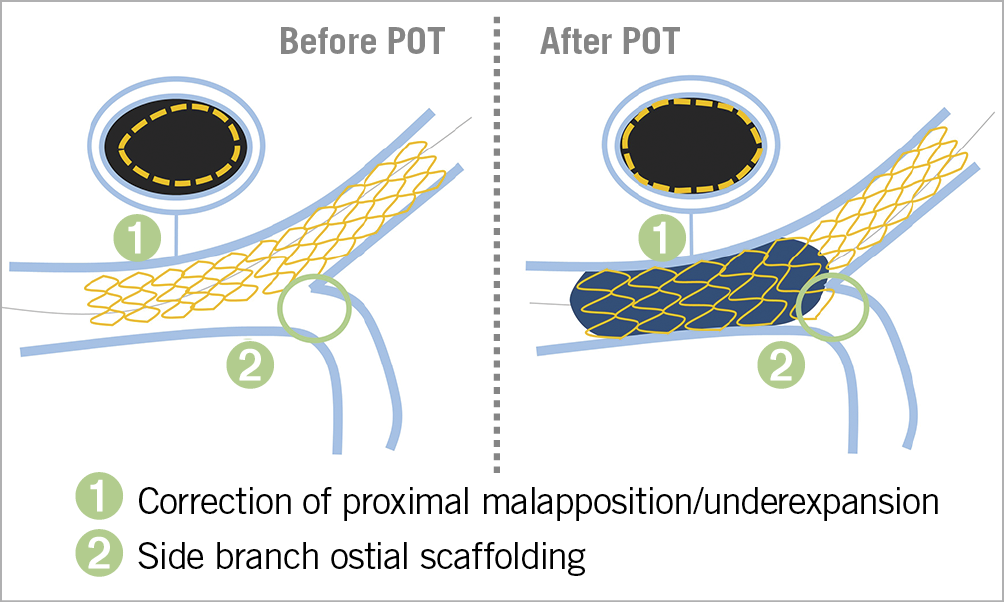
Figure 2. Proximal optimisation technique (POT). The technique and effects of POT with regard to both the proximal segment of the left main bifurcation and the ostium of the side branch. POT: proximal optimisation technique
Following POT, an unsatisfactory result in the SB may necessitate further intervention, but routine KBI after main vessel stenting has not been shown to improve outcomes in a randomised trial involving non-LM bifurcations42. Observational data in patients with distal LM bifurcation lesions treated with the provisional strategy reaffirmed that final KBI is associated with neither benefit nor harm if routinely applied43,44. The mechanics of KBI, such as recentring of the carina and overexpansion at the point of confluence and in the proximal main vessel, seem to have the most clinical benefits when performed in the course of a two-stent bifurcation PCI45. Although a recent sub-analysis of the EXCEL trial did not show any benefits of final KBI in patients treated with either one or two stents46, there is convincing historical and contemporary evidence47 (Table 3) that confirms the value of final KBI if a bifurcation is treated with two stents and it is now recognised as a procedural quality marker when performing two-stent bifurcation PCI35. Of note, the use of non-compliant (NC) balloons, sized 1:1 according to the distal reference, and with short proximal overlap, has been associated with improved clinical outcomes45,48. Future research will further clarify the clinical impact of the procedural interaction between POT and KBI47.
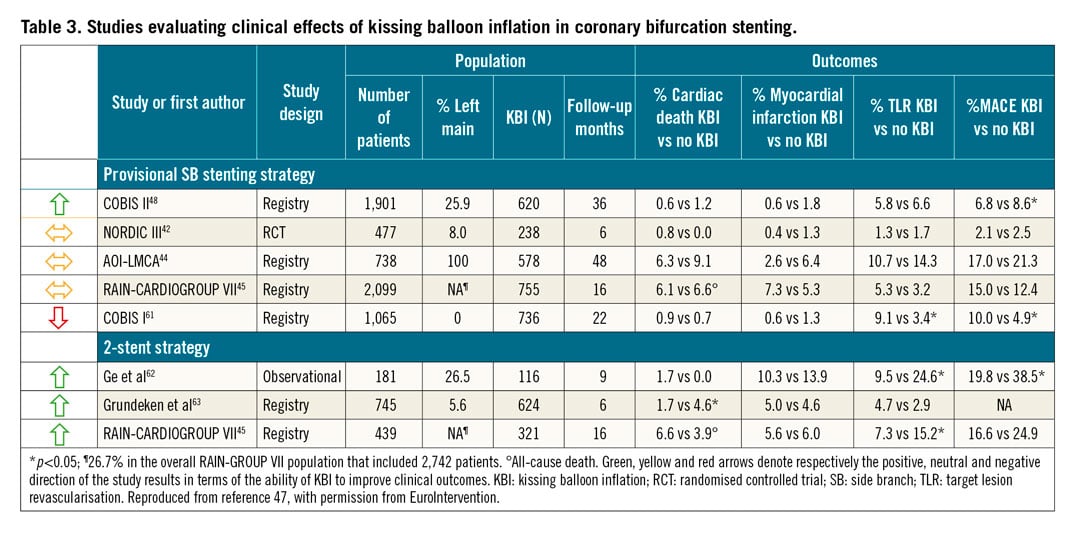
WHICH TWO-STENT TECHNIQUE?
If, based on the underlying anatomical complexity, an upfront two-stent strategy is decided, the most commonly used techniques are T- or TAP, culotte and DK crush. Only one randomised study with a direct comparison of culotte and DK crush for LM disease has been published (Table 2). In the DKCRUSH-III trial49, the culotte technique was shown to be inferior to the DK crush technique due primarily to an increased rate of one-year TVR (11.0% vs 4.3%, p<0.05). The rate of in-stent restenosis (ISR) in the CX artery was significantly more frequent in the culotte group (12.6% vs 6.8%, p<0.05). The benefit of the DK crush over the culotte technique was compounded in the presence of a wide bifurcation angle (≥70°) and increased overall disease complexity (SYNTAX score ≥23)49. These data notwithstanding, the decision on which two-stent technique to perform should also include considerations regarding local and individual operator’s expertise. It should be kept in mind that the convincing results of the DKCRUSH-V trial relied, at least in part, on the fact that participating operators had to be experienced with the DK crush technique, as was formally confirmed by submitting 3-5 cases to be reviewed by the trial’s steering committee before entering the study31.
OPERATOR’S EXPERIENCE
Current clinical practice guidelines recommend that LM PCI be performed by operators with an annual experience of 25 LM PCI cases2. This recommendation is based on several reports of the association between the annual LM PCI volumes performed and clinical outcomes. In a single-centre registry including 1,948 patients who underwent PCI for unprotected LM disease, the rate of cardiac death was significantly lower at 30 days and three years if the procedure had been performed by an operator with an annual LM PCI volume of ≥15 procedures as compared with operators from the same centre who performed fewer than 15 LM PCI procedures per year50. A more recent study involving 6,724 LM PCI procedures from the British Cardiovascular Intervention Society National Database showed a close relationship between the annual operator’s LM PCI performance volume and the one-year survival rate, with the lower threshold of ≥16 LM PCI cases/year improving survival as compared with lower annual performance volumes51.
INTRACORONARY IMAGING GUIDANCE
Several pooled analyses of observational data demonstrated improved outcomes if IVUS was used to guide the procedure in the LM, with the benefit being most evident in patients with complex lesions52,53. More recently, data from the British Cardiovascular Intervention Society National Database including 11,264 unprotected LM PCIs, showed an increase in the use of intracoronary imaging from 30.2% in 2007 to 50.2% in 201454. Importantly, the use of intracoronary imaging was associated with improved one-year mortality. Although intracoronary imaging guidance is further supported by the results of the randomised ULTIMATE trial, which suggested clinical benefits of the routine use of IVUS55, its widespread application is hampered not only by the costs, but also by the lack of clear-cut clinical outcome-related markers of procedural success. An important step in this direction is a recent study, which evaluated the effects of standardised IVUS interrogation pre- and post-stenting versus non-standardised IVUS use. Interestingly, having a clear algorithm of predefined optimisation criteria on IVUS, such as a systematic assessment of stent expansion and apposition of the result at stent edges and longitudinal deformation, was shown to be superior to a non-standardised utilisation of IVUS in LM PCI56. Most of the described evidence stems from IVUS-based studies, with new emerging data signalling feasibility for the use of optical coherence tomography (OCT) in the LM. The recently published LEft Main Oct-guided iNterventions (LEMON) study, showed that OCT-derived information regarding stent expansion, apposition and dissection at stent edges led to a change in procedural strategy in 26% of the studied cases of LM PCI57. Ongoing studies such as the randomised OCTOBER trial will assess the specific utility of OCT, with its high resolution to guide the bifurcation stenting process beyond the traditional imaging optimisation criteria, to include important procedural steps such as wire positioning58.
Conclusion
The presented evidence may be integrated into a common clinical treatment pathway (Figure 3) for patients diagnosed with LM disease, which may rely on the following principles: first, in the case of angiographic ambiguity, intracoronary imaging and physiology can be used to decide whether revascularisation can be deferred. Second, if revascularisation is indicated, anatomical and clinical characteristics, as well as local expertise in percutaneous versus surgical procedures should be evaluated before deciding between PCI or CABG as the preferred strategy. Third, if PCI is chosen, the relative merits of a provisional versus upfront two-stent strategy should be assessed. In the great majority of cases the current evidence base favours the initial provisional single-stent strategy, even in true distal LM bifurcations without extensive and complex disease in the CX. In complex distal LM bifurcation lesions, however, if there is an extensive involvement of the CX, DK crush may be the preferred strategy in the hands of experienced operators. Finally, the widespread use of a standardised protocol for intracoronary imaging guidance is advisable to improve the outcomes of LM PCI.
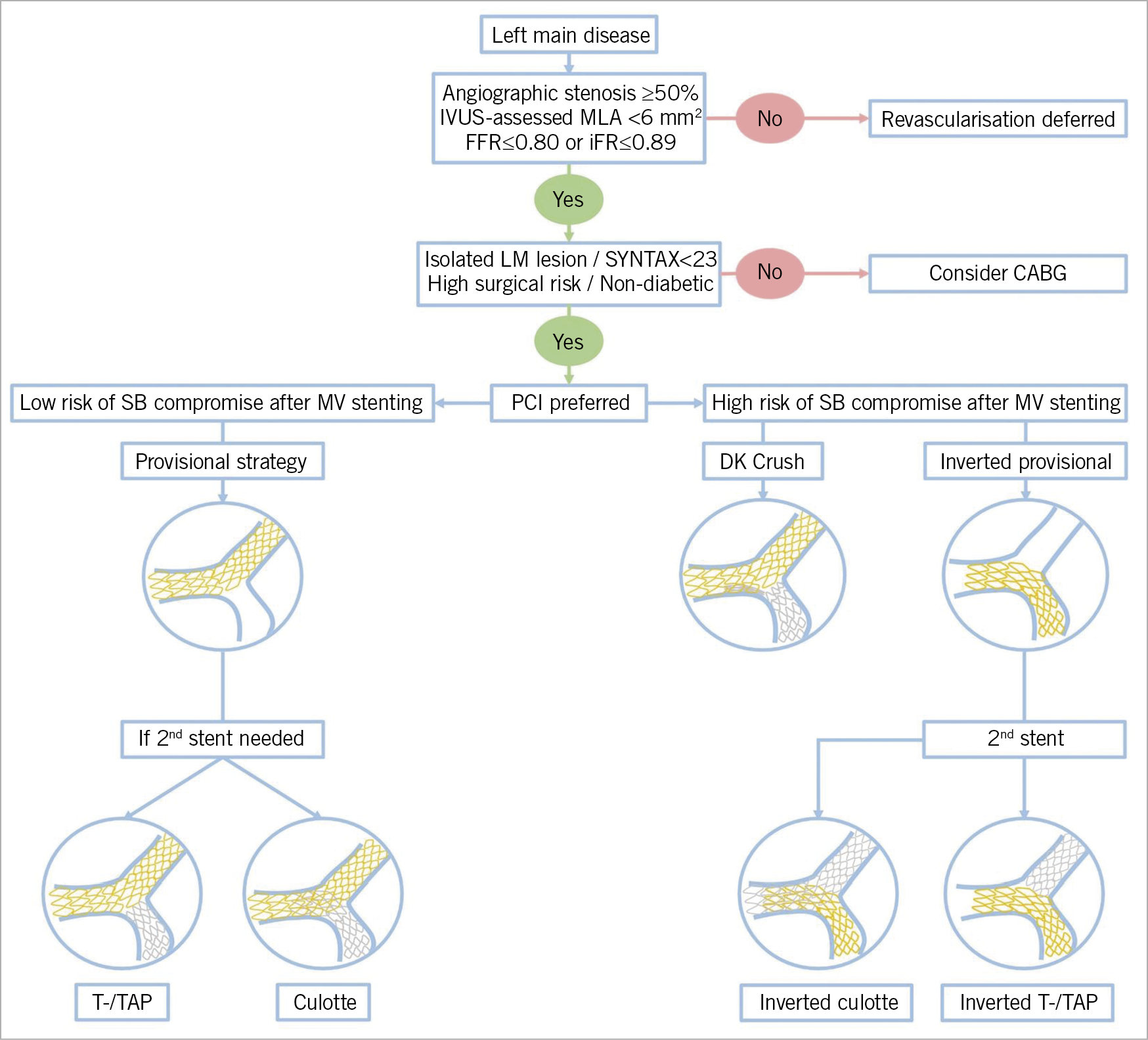
Figure 3. Algorithmic approach to left main treatment. After establishing the significance of an LM lesion, the first step is to decide between percutaneous and surgical revascularisation strategies. If a percutaneous intervention is preferred, the key factor in deciding between the provisional strategy and the potential upfront two-stent technique relies on the assessment of the risk of SB loss following main vessel stenting. If the risk is high, DK crush or an inverted provisional strategy, treating the SB first before proceeding with the 2nd stent, is warranted. In cases with no disease at the SB ostium and/or if the risk of compromising the SB is low, a provisional strategy with crossover main vessel stenting is preferred. If necessary, the provisional single-stent strategy is extended to 2nd stent implantation following the steps of the T-/TAP or culotte technique.
Conflict of interest statement
The authors have no conflicts of interest to declare.

