Introduction
Surgical aortic valve replacement (SAVR) is currently the treatment of choice for patients with severe pure native aortic regurgitation (AR) requiring intervention1. However, there remains a therapeutic dilemma for patients with severe AR, in particular those with reduced left ventricular function, as studies have shown that postoperative outcomes are much worse for this group of patients2. These patients are mostly considered high-risk or inoperable and thus, there remains a gap in current management for patients with symptomatic severe AR who are at high risk for surgery.
Transcatheter aortic valve replacement (TAVR) was originally indicated as a treatment for patients with severe symptomatic aortic stenosis and has been shown to have comparable results to SAVR3. Since then, TAVR has been used more and more for off-label indications such as valve-in-valve and native AR interventions. Recently, the US Food and Drug Administration has approved the use of the Medtronic Evolut™ (Medtronic, Minneapolis, MN, USA) TAVR platform in bicuspid aortic valve disease, further expanding the use of TAVR in younger, lower-risk patients4. Pure severe native aortic regurgitation is defined as the presence of severe AR not associated with significant aortic stenosis (AS) or failed surgical valve. TAVR has emerged as a potential treatment option for this patient population and it may offer better outcomes than optimal medical treatment for inoperable severe AR patients5.
Although aortic regurgitation has a higher prevalence in the elderly Asian population as compared to Western patients6, it remains a relatively under-researched field. Moreover, anatomic differences in the Asian population (including eccentric valvular calcification and a smaller aortic valve annulus) may pose unique challenges to TAVR. Hence, this systematic review aims to study the characteristics and outcomes of TAVR performed in an Asian population with pure native aortic regurgitation in the hope of better aiding clinicians to consider it as a possible intervention for their patients.
Methods
LITERATURE SEARCH
Five databases were searched electronically in April 2020: PubMed, Embase, Scopus, Web of Science and Cochrane CENTRAL. Study selection, data extraction, risk of bias assessment and quality assessment were conducted by two independent reviewers (E.L. Soong, Y.J. Ong). Data management and synthesis was done using Rayyan QCRI (Rayyan Systems Inc, Cambridge, MA USA). The following Boolean operators were used: (“aortic regurgitation” OR “aortic valve regurgitation” OR “aortic insufficiency” OR “aortic valve insufficiency” OR “AR” OR “AI”) AND (“transcatheter aortic valve implantation” OR “transcatheter aortic valve replacement” OR “TAVI” OR “TAVR”).
STUDY SELECTION
Studies were selected if they met the following inclusion criteria:
1. patients with native aortic regurgitation (no concomitant aortic stenosis)
2. studies from Asian centres, or studies in non-Asian centres specifically reporting outcomes of Asian patients
The exclusion criteria were as follows:
1. prior intervention to the aortic valve (prosthetic or repaired aortic valve)
2. concomitant procedure(s) at the time of TAVR
3. paediatric population (defined as patients aged <18 years old)
4. no report of mortality or morbidity
Randomised controlled trials (RCTs), observational studies (prospective, retrospective, case-controlled) and case reports were included. Articles without any primary data such as abstracts, systematic reviews, meta-analyses, comments, letters to the editor, and expert opinions were excluded.
To prevent duplicate reporting of patient cohorts, whenever a similar co-author was identified between abstracts, the publication with the greater number of patients was included unless the patient population was clearly distinct between studies after full-text review.
RISK OF BIAS AND QUALITY ASSESSMENT
The risk of bias of the included studies was assessed independently by two authors (E.L. Soong, Y.J. Ong) and is presented in a risk of bias table (Supplementary Table 1-Supplementary Table 3). Risk of bias for the cohort study was assessed using the Newcastle-Ottawa Quality Assessment Scale (NOS)7 while risk of bias for the case series was assessed using the Institute of Health Economics Quality Appraisal Checklist8. Case reports were assessed using the scale developed by Murad et al9.
DATA EXTRACTION
Data on key baseline characteristics and outcomes were extracted independently by two reviewers (E.L. Soong, Y.J. Ong) and stored on pre-made proformas. Outcomes of interests included all-cause mortality, cardiovascular mortality, myocardial infarction, stroke or transient ischaemic attack, major or life-threatening bleeding, major vascular complication, acute kidney injury (≥ stage 2), permanent pacemaker implantation, infective endocarditis, paravalvular leak, device migration and valve thrombosis.
STATISTICAL ANALYSIS
For prevalence and continuous outcomes, we performed meta-analyses of proportions using the Freeman-Tukey transformation, and mean differences respectively. The random-effects model was performed to pool the outcomes. Meta-analyses were performed using OpenMeta[Analyst]. Subgroup analysis was conducted by stratifying studies according to: (1) type of valves (J-Valve™ [Jiecheng Medical Technology Co, Suzhou, China] vs CoreValve® [Medtronic]) and (2) mean logistic EuroSCORE (<20 and >20) for device success and all-cause mortality respectively. All continuous variables were presented as means±standard deviation for parametric variables and medians with interquartile range (IQR) for non-parametric variables. Categorical variables are described as number (%). The I2 statistic was used to assess heterogeneity and a value of I2=25%-50% was considered mild, 50%-75% as moderate and >75% as severe. A p-value of <0.05 was considered statistically significant in all cases.
As this was a systematic review and meta-analysis based on published data, ethical review and specific informed consent were not required.
Results
LITERATURE SEARCH AND STUDY CHARACTERISTICS
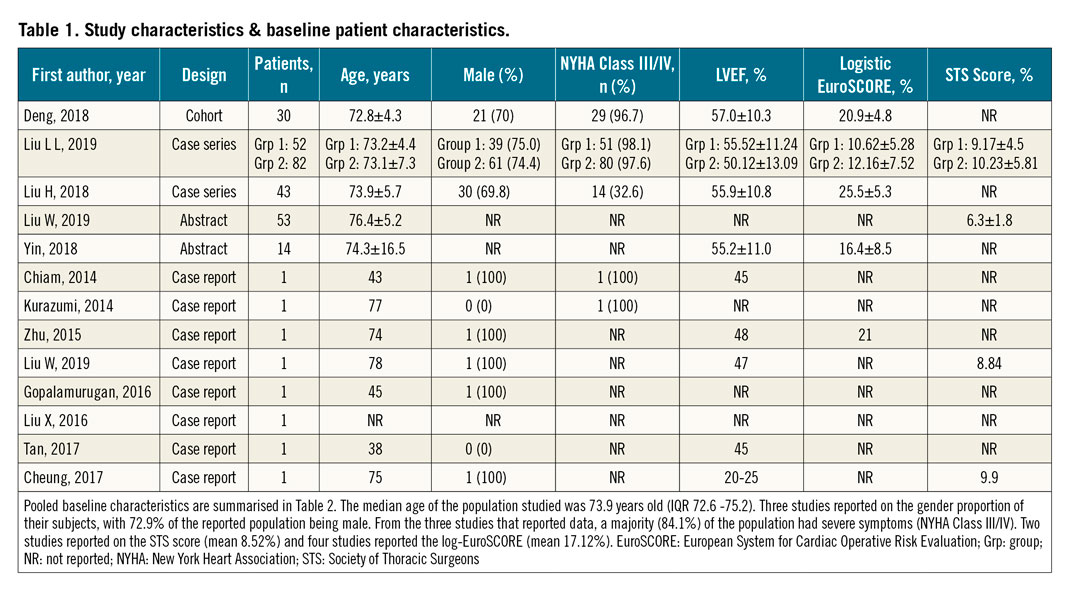
The initial search revealed a total of 9,727 potential articles. After exclusion, 13 reports remained for analysis (three full texts, two conference abstracts and eight case reports). The PRISMA flow chart (Figure 1, Supplementary Table 4) gives an overview of the literature search. The studies (excluding case reports) included a total of 274 patients undergoing TAVR for native AR and were generally of moderate quality, with risk of selection bias and reporting bias (Supplementary Table 1-Supplementary Table 3). One study had a multicentre design while the remaining four were single-centre. The mean age of the patients ranged from 72.6 to 75.2 years, and the mean logistic EuroSCORE ranged from 10.89 to 23.35. Relevant individual study and baseline patient characteristics are displayed in Table 1. The pooled baseline characteristics are shown in Table 2. Case reports were analysed separately.
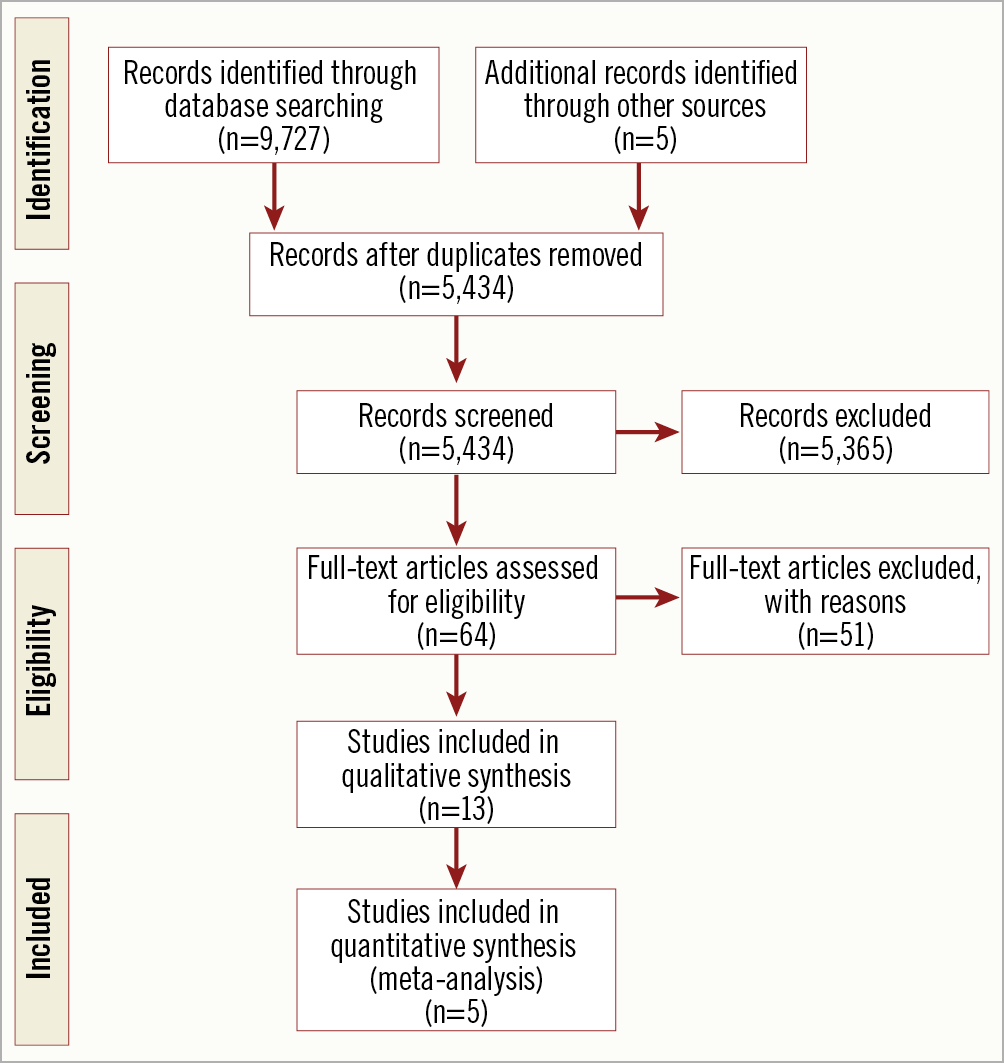
Figure 1. PRISMA flow diagram.
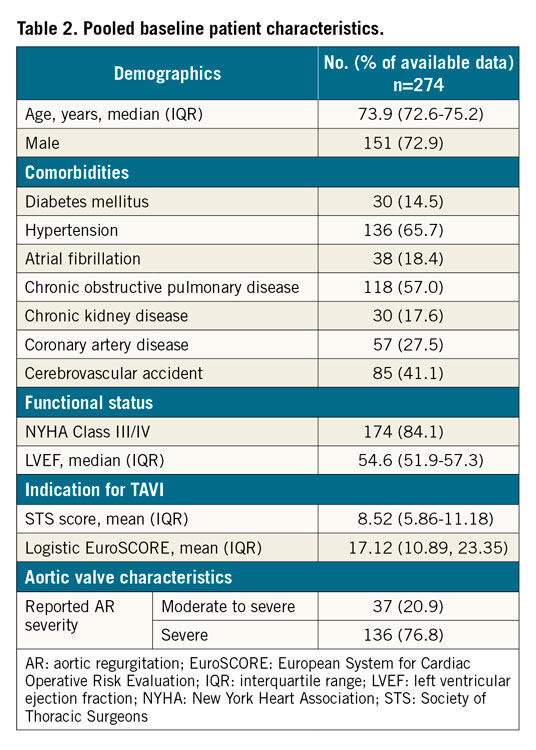
Only two studies10,11 reported the quantitative assessment of AR severity – of which most of the patients (76.8%) had severe AR while 20.9% of the patients had moderate AR. Only one full report12 recorded the AR aetiology of its included patients, with the majority of cases due to degeneration (72.1%) with rheumatic heart disease (23.2%) and bicuspid aortic valve (4.7%) accounting for the remainder of the cases. Two case reports13,14 also recorded AR aetiology, both secondary to infective endocarditis. None of the studies or case reports reported other aortic valve characteristics such as valve calcification or shape.
PROCEDURAL INFORMATION
The reason for choosing TAVR over SAVR was mentioned in four studies and three case reports, with all quoting high or prohibitive surgical risk or severe AR as the main reason SAVR was declined. Four full studies used J-Valve with a transapical approach while one full study used CoreValve with a transfemoral approach. The mean valve size chosen for the procedure based on the available data from three full studies was 26.3 mm (IQR 26.0-26.6). The device used and valve size varied in the case reports based on the specific patient’s requirements. Breakdown of procedural information for each study can be found in Table 3.
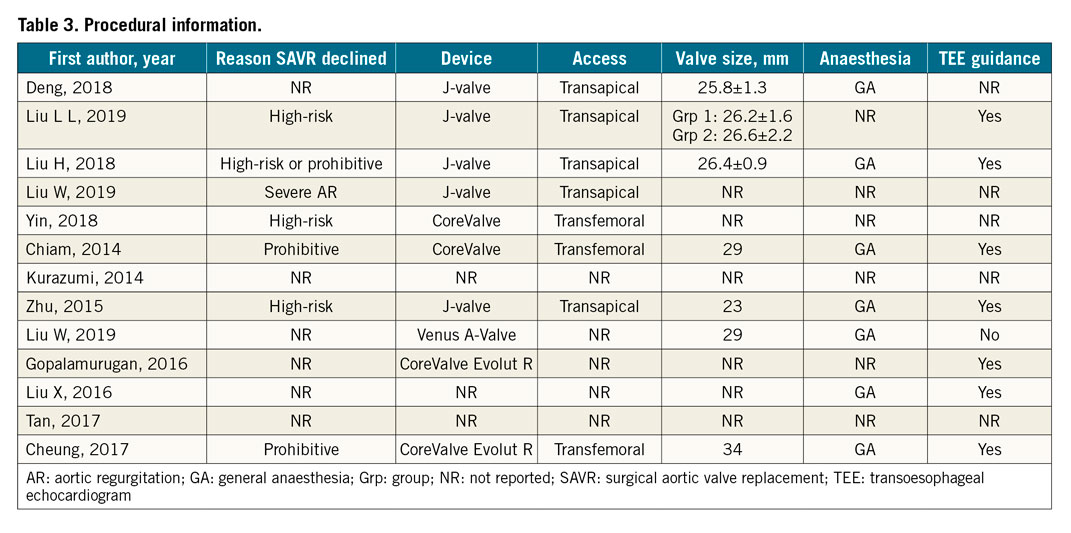
Of note, two full studies and five case reports reported the use of general anaesthesia for TAVR, while the other three full studies and three case reports did not specify the type of anaesthesia used. Additionally, two studies and five case reports used transoesophageal echocardiogram (TEE) guidance in the procedure. No information on oversizing of the valve or rapid pacing during the procedure was available.
OUTCOMES AND META-ANALYSIS
Details on the clinical outcomes for each study can be found in Table 4. Results of the meta-analysis are summarised in Figure 2 and the Central illustration. Detailed forest plots outlining the effect size of each study are given in Supplementary Figure 1, Supplementary Figure 2.
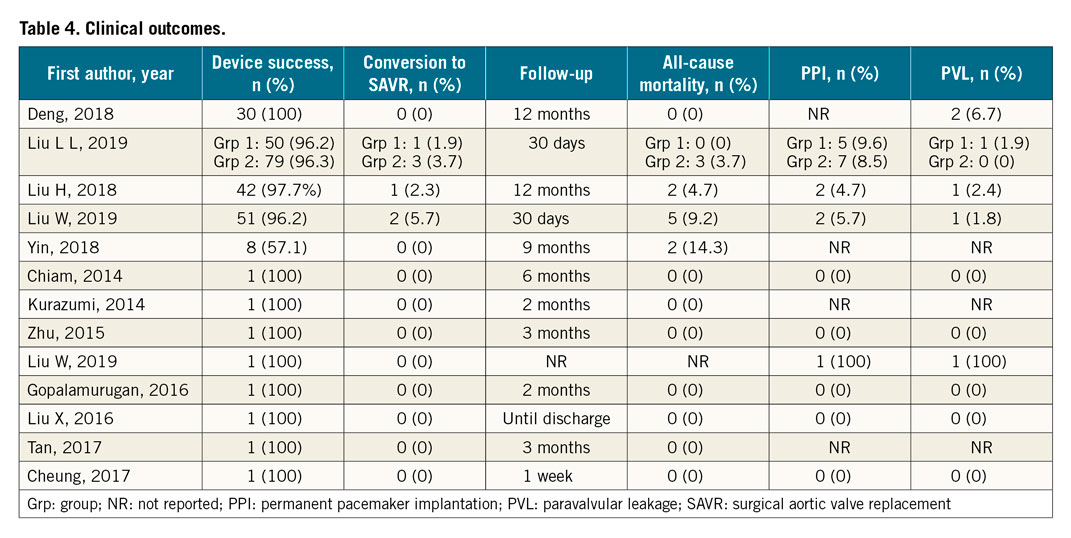
Figure 2. Bar graph showing pooled incidence of each clinical outcome, along with 95% confidence interval. PPI: permanent pacemaker implantation; PVL: paravalvular leakage; SAVR: surgical aortic valve replacement
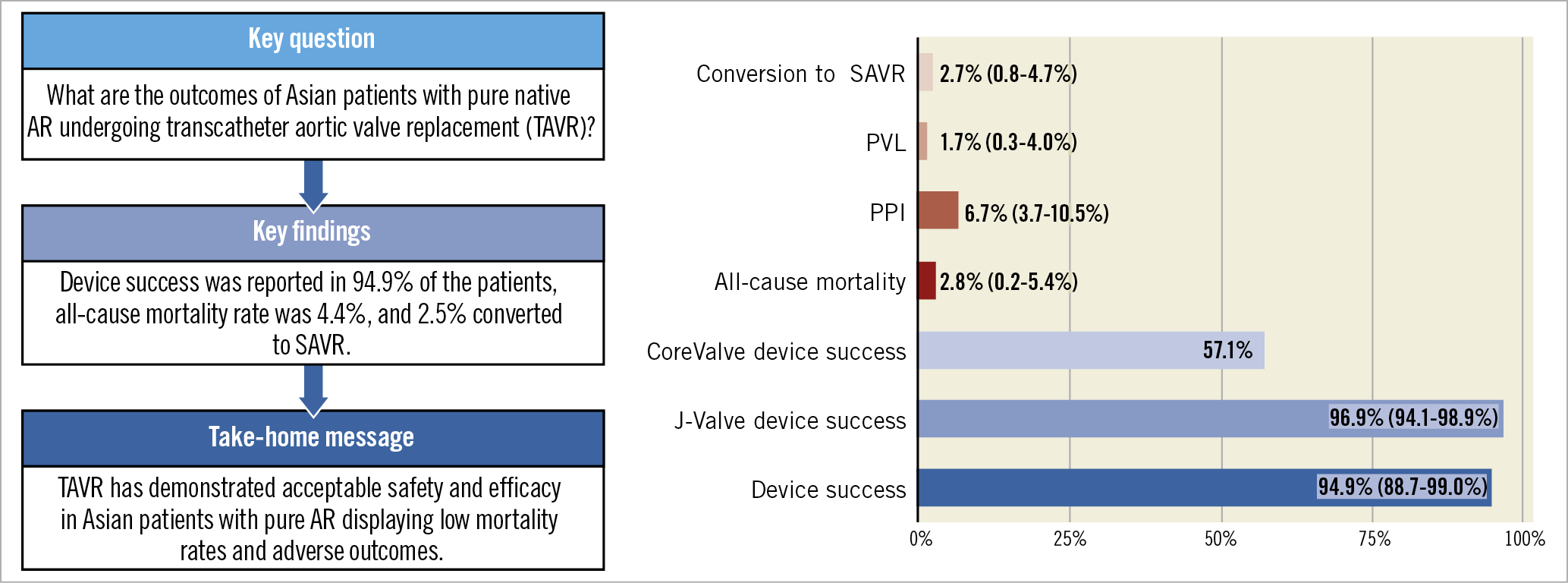
Central illustration. TAVR for pure native AR in the Asian population showed acceptable safety and efficacy outcomes. AR: aortic regurgitation; PPI: permanent pacemaker implantation; PVL: paravalvular leakage; SAVR: surgical aortic valve replacement
DEVICE SUCCESS
All five studies reported device success, which ranged from 96.2% to 100% with a summary estimate of 94.9% (88.7%-99.0%; [I2=66.45]), and moderate statistical heterogeneity (Supplementary Figure 1). On subgroup analysis by valve device used, 252 (96.9%) out of 260 patients receiving a J-Valve had device success, compared to 8 (57.1%) out of 14 patients receiving a CoreValve.
Of the eight patients who underwent TAVR with J-Valve and experienced device failure, six were converted to SAVR, one was successfully implanted with another J-valve and the reason for device failure was not stated for one patient. Of the six patients who underwent TAVR with CoreValve and experienced device failure, five had severe paravalvular leakage (PVL) and required a second valve implantation while one had moderate PVL.
CONVERSION TO OPEN SURGERY
Four studies reported the rate of conversion to open surgery, varying from 2.0% to 3.7%, with a pooled estimate of 2.7% (0.8%-4.7%; [I2=0%]). Among the eight patients who were converted to open surgery, seven conversions were done intraoperatively. Six of these were due to valve migration, while one was due to severe PVL. The remaining patient had moderate PVL postoperatively and developed congestive heart failure one-week post-op, thus requiring conversion to SAVR. Among the six patients with valve migration, no data on oversizing for their procedure was reported.
ALL-CAUSE MORTALITY
Four studies reported all-cause mortality and the 30-day mortality ranged from 2.2% to 9.4%, with a pooled estimate of 2.8% (0.2%-5.4%; [I2=35.21%]) and mild between-study heterogeneity across three studies (Supplementary Figure 2). The nine-month mortality was reported as 14.3% (2 out of 14 patients) in one case series15, and the one-year mortality was 4.7% in another case series of 43 patients11. Of the 12 patients who died, one patient had been converted to SAVR due to device migration and had a stroke one-month post-op, three were due to cardiac causes, one due to digestive tract haemorrhage, three due to infection and four were not stated.
Subgroup analysis revealed that there was no significant difference in the mortality rate between subgroups of patients with logistic EuroSCORE <20% (n=73) (3.1%) and those with logistic EuroSCORE >20% (n=148) (3.2%).
PARAVALVULAR LEAKAGE
Four studies reported the rate of paravalvular leakage (moderate to severe), varying from 1.8% to 2.4%, with a pooled estimate of 1.7% (0.3%-4.0%; [I2=0%]).
PERMANENT PACEMAKER (PPM) IMPLANTATION
Three studies reported the rate of post-procedural PPM implantation, varying from 4.7% to 5.6%, with a pooled estimate of 6.7% (3.7%-10.5%; [I2=0%]).
Discussion
JUSTIFICATION OF TAVR IN NATIVE AR
Current guidelines suggest that native AR patients who are symptomatic or with left ventricular ejection fraction (LVEF) <50% should undergo surgical aortic valve replacement (SAVR), while similar patients who have contraindications for SAVR are to be treated conservatively with medical therapy1. However, there has been little research done on the efficacy of medical therapy in the treatment of AR, and that which has been done has come to conflicting conclusions on its effectiveness16. Moreover, patients with severe AR (NYHA Class III or IV) on medical treatment have a mortality rate of nearly 25% a year17. This shows that there exists an unmet clinical need for patients with inoperable severe AR.
Given that multiple studies have shown that TAVR has better outcomes than medical treatment for patients with inoperable aortic stenosis18, there is growing interest in a similar trajectory for AR patients with high or prohibitive surgical risk. From the studies that we have analysed, the all-cause mortality rate is comparable to that of the PARTNER trial assessing TAVR for aortic stenosis19 as well as various studies on SAVR in AR patients2. This indicates that despite unique challenges in its implementation that will be further elaborated on below, TAVR can still be considered as an alternative option for well-selected patients with acceptable efficacy and safety data.
CHALLENGES OF TAVR IN NATIVE AR
Native AR patients remain a challenge for TAVR procedure. While most severe AS is due to calcification and manifests later in life, the diverse aetiologies of AR result in more complex and diverse anatomy. In our included studies that reported on AR aetiology, a majority were due to degeneration but rheumatic heart disease, bicuspid aortic valve and infective endocarditis were also noted causes. In severe pure AR, the absence of annulus calcification makes device anchoring and stabilisation during deployment more challenging, increasing the risk of post-TAVR paravalvular leak and device embolisation20. AR is also frequently associated with dilatation of the aortic root which is usually accompanied by an extremely large annulus which exceeds most commercial TAVR valve devices21. This issue is often resolved by oversizing the valve for better anchoring to the annulus to make up for minimal calcification, thus preventing valve embolisation. Unfortunately, oversizing the valve may increase the risk of annular rupture and atrioventricular block. These anatomic challenges are reflected in the outcomes of our studies, in which paravalvular leak, permanent pacemaker implantation and conversion to SAVR due to valve migration are the most commonly reported complications. Due to limited data on annulus calcification, dilatation and shape, it is difficult for us to correlate whether these anatomical findings are strongly related to the procedural outcomes. Nonetheless, the rate of these complications is acceptable, and potentially with increased experience and future developments of new-generation valves specifically for native AR, the rate of these complications can be decreased.
TYPES OF VALVES FOR TAVR IN NATIVE AR: J-VALVE VS COREVALVE
Valve devices have evolved significantly since the first TAVR procedure was performed in 2002 and can generally be split into first- and second-generation devices. In the Asian studies, the most popular valve devices used to treat native AR were CoreValve (a first-generation Medtronic self-expandable valve) and J-Valve. This is different from other regions such as Europe where the JenaValve ([JenaValve Technology, Munich, Germany], a valve made specifically to treat native AR) has seen much higher use than the J-Valve. Subgroup analysis of our included studies revealed that the use of the newer-generation valves such as J-Valve have a much higher success rate compared to CoreValve, a trend that is comparable to other international studies on TAVR in AR using different generation valves21, with first-generation valves having device success ranging from 54% to 79% and second-generation valves having device success ranging from 81.1% to 100%. With the available evidence in mind, it may be advisable for clinicians to consider second-generation valves such as the Medtronic Evolut R or other valves made specifically for native AR, such as the J-Valve, for this patient population. The much higher device success rate may have to do with the J-Valve’s clip-based design over the native aortic valve leaflet alleviating the dependency on aortic annular calcification, while the Medtronic Evolut R has the benefit of being recapturable and repositionable.
INTER-ETHNIC DIFFERENCES IN TAVR
Racial differences in the vessel anatomy, representation in clinical trials and overall utilisation have been observed in previous studies22. In Asian populations, the uptake of TAVR has been slow, with fewer than 10% of TAVRs worldwide performed in Asia despite its larger population23. There are few publications on the outcomes of TAVR in Asian populations, and no randomised controlled trials to date. Due to anatomical differences, TAVR has caused particular concern in the Asian population. Compared to Caucasians, Asians have a smaller aortic annulus area, a smaller left coronary cusp diameter, and a lower height of the left coronary ostia24. One Korean study found that one-third of Asian patients have both a lower height of left coronary ostia and smaller sinuses of Valsalva, which increases the risk of coronary obstruction after TAVR25. Although a smaller aortic annulus theoretically reduces the risk of perivalvular leaks, it is unknown if the benefit is evident in Asian patients with AR. Observational studies and large registries showed that clinical outcomes of TAVR for aortic stenosis in Asian patients are generally good, with a procedural success rate of 97.5% and 30-day mortality rate of 2.5% in an international Asian registry26. A prospective, multicentre, non-randomised trial in Japan found that clinical outcomes after TAVR in severe aortic stenosis were similar to a single-centre European cohort27. This study showed however, that the use of TAVR for AR in the Asian population is limited, particularly for Asians outside of China, and the gap in evidence may impede the adoption of TAVR in this large population.
Limitations
This systematic review has a number of limitations. First, given the novel off-label nature of TAVR as an intervention for AR patients especially in the Asian population, the pool of studies that we can analyse is currently very limited. Most of the studies identified were Chinese, therefore many of the other Asian countries and ethnicities were under-represented. Furthermore, only one study was a cohort study with a control group, the others being case series and case reports. None of the studies included had research data or clinical endpoints adjudicated through independent core labs, which exposed the results to observer and confirmation bias that may lead to overestimation of benefits and underestimation of complications. Selection bias was a factor as we were only able to find conference abstracts for six of our included studies. Second, reporting bias was also a factor, as some studies did not fully report on all the clinical outcomes we sought to collect data for, and some did not adhere to the current Valve Academic Research Consortium (VARC) criteria. More pressingly, many studies failed to provide information on certain baseline patient characteristics and procedural information which may have had an impact on the clinical outcomes of the studies. It should be noted that few of the studies recorded the AR aetiology of their included patients, and none recorded on aortic valve calcification. This is of interest to us as the absence of calcification in AR makes it more challenging to implant currently available valve devices by TAVR. There was also limited data on the procedural characteristics such as the use of rapid pacing, oversizing of valve, TEE guidance during TAVR, and other valve characteristics such as aortic valve shape, and annular dilatation – all of which could influence the success rate. More information is required to correlate these factors with post-procedural outcomes and success. Third, our subgroup analysis was based on indirect comparisons between separate studies rather than on consecutive patients in a single centre using the same inclusion criteria. Finally, there are many devices, techniques and modes of access available for TAVR and the outcomes may differ based on operator experience, which may contribute to heterogeneity between studies, although statistical heterogeneity was small for most outcomes in this meta-analysis. Most of the studies included in this review were performed early in the adoption of TAVR, particularly for AR. Based on previous long-term studies that explored the trends in complications and outcomes of TAVR over time28, it would be expected that with better case selection, improved procedural techniques and increased experience, outcomes of TAVR for AR would improve. Hence, the outcomes from our systematic review may be less readily applicable to the broader contemporary population.
Conclusions
In this study, TAVR has demonstrated acceptable safety and efficacy in Asian patients with native AR, displaying low mortality rates and adverse outcomes. This is especially pertinent in AR patients with high or prohibitive surgical risk who are not candidates for SAVR, which is the current recommended intervention. Among Asian treatment, J-Valve is the preferred second-generation device and CoreValve the preferred first-generation device; other devices were used on a case-by-case basis. More studies, ideally randomised controlled trials, need to be performed in order to come to more solid conclusions.
Impact on daily practiceTAVR has demonstrated acceptable safety and efficacy in Asian patients with native AR, displaying low mortality rates and adverse outcomes. TAVR may be a suitable alternative in native AR, particularly in patients with high or prohibitive surgical risk for SAVR. However, additional studies are needed to confirm these findings. |
Funding
C-H Sia was supported by the National University of Singapore Yong Loo Lin School of Medicine’s Junior Academic Faculty Scheme.
Conflict of interest statement
The authors have no conflicts of interest to declare.
