Introduction
Aortic stenosis (AS) is one of the most common chronic, progressive heart valve disorders. The global prevalence of calcific aortic valve (AV) disease has increased more than 4-fold over the last 30 years. In 2019, AS afflicted over 9 million individuals worldwide, exhibiting an age-standardised prevalence of 116.3 cases per 100,000 population1. Notably, East Asia experienced a staggering 47-fold increase in prevalence. Ageing is one of the main drivers of AS in Western and high-income Asian countries23, and the global prevalence of AS is expected to increase further in the coming years due to the ageing population in many countries14. According to current guidelines, treatment is recommended in patients at later stages of severe AS, when the risk of operative mortality is outweighed by the significantly elevated risk of mortality from the disease itself5, emphasising valve replacement as a lifesaving measure for severe AS cases67.
Treatment options for severe AS include surgical AV replacement (SAVR) and transcatheter AV replacement (TAVR)8. The treatment choice is made after weighing up an individual patient’s lifetime risks and benefits. In general, SAVR is recommended for younger patients at low surgical risk, while transfemoral TAVR is recommended for more elderly patients and those who are at high surgical risk89. Either SAVR or TAVR may be appropriate for other patients, depending on their clinical characteristics.
The use of TAVR is increasing in many countries, although there is wide variation21011. Uptake of TAVR was initially slower in Asian countries than in many Western countries, although it is now a well-established treatment strategy in the region12. China has a population of more than 1.3 billion people, 16% of whom are aged ≥60 years13. The increase in ageing of the population means that the burden of degenerative heart valve disease in the country is also increasing14. The uptake of TAVR has grown in recent years in China, and several locally manufactured valves are available in addition to more established, internationally approved valves12.
This paper aims to describe the epidemiology and treatment of AS in China, with a particular focus on the current status of TAVR for treating AS in an ageing population. The epidemiology, patient characteristics and treatment of AS in China are described. A systematic review has been performed to summarise the clinical outcomes of studies of transfemoral TAVR in the Chinese population.
Epidemiology of aortic stenosis in China
The prevalence of AS in China exhibits some uncertainty, although it appears lower than in Western countries15. In a large community-based study involving 14,618 people aged ≥35 years, the overall prevalence of AV disease (all types) in its Chinese population was 5.81%, with a marked increase in prevalence seen with increasing age, reaching 18.09% in those aged 65-74 years and 28.26% in those aged ≥75 years16. A cross-sectional national survey involving 34,994 individuals aged ≥35 years reported an overall AS prevalence of 0.7%, with the highest rates seen in those aged ≥65 years (1.5% among those aged 65-74 years and 3.4% among those aged ≥75 years)14.
Smaller community-based studies have reported an AS prevalence of 0.13% among people aged ≥60 years (n=3,948; mean age 67 years)17 and 1.1% among people aged ≥65 years (n=3,538; mean age 72 years)18. A retrospective analysis of 287,556 echocardiography-referred patients found AS in 1.26% of patients aged 65-84 years and in 1.76% of those aged ≥85 years, with severe AS found in 0.43% and 0.47% of these age groups, respectively15.
Although rheumatic AS remains common in China, AV calcification is the most common cause of AS among people aged ≥65 years (43.70% of cases of AS at age 65-84 years and 79.75% in those aged ≥85 years15. In the hospital referral study discussed above, calcific AS was detected in 0.60% of patients aged ≥65 years, 0.55% of those aged 65-84 years and 1.41% of those aged ≥85 years15. A recent epidemiological study found that the prevalence of calcific AS was 4.1% (59/1,423) among those aged <60 years, 10.3% (131/1,278) among those aged 60-70 years and 21.9% (80/366) among those aged >70 years19.
Therefore, considering China’s population size, AS represents a substantial healthcare burden, which is likely to increase as the population ages.
Clinical characteristics of AS patients in China
In the China-DVD Study, a nationwide survey of hospitalised patients aged >60 years with clinically significant (moderate or severe) valvular heart disease in China, the median age of patients with AS (n=419) was 69 years (interquartile range 65-76), 36.8% were female, and 92.4% were symptomatic20. Comorbidities were common, including hypertension (49.4%), smoking (38.4%), angina (33.2%), coronary artery disease (31.5%) and diabetes (21.0%)20. A study on low-risk patients (mean age 72.9 years; 65.1% male) undergoing TAVR with self-expanding transcatheter heart valves (THVs) from 36 centres in China (National Transcatheter Valve Therapeutics Registry [NTCVR]) revealed higher comorbidities in patients with tricuspid AV (TV) than bicuspid AV (BAV) morphology. These comorbidities included hypertension (52.5% vs 44.1%), smoking (26.9% vs 21.0%), diabetes (23.8% vs 13.5%), and chronic lung disease (10.6% vs 8.7%)21.
According to the China Aortic valve tRanscatheter Replacement registrY (CARRY; n=2,097), the mean age of patients with symptomatic severe AS sent for TAVR screening was 73.2±7.6 years, and 58.4% were male22. Patients with symptomatic severe AS in China had a higher burden of aortic leaflet calcification (approximately 680 mm3) compared with other countries (approximately 350 mm3)22.
Analysis of data from 438 patients with severe AS who underwent TAVR with the SAPIEN 3 (Edwards Lifesciences) valve showed that the mean age of patients was 73.7±8.6 years, 55% were male, and 35.2% had severe aortic leaflet calcification23.
A high proportion of AS patients in China have a BAV. A systematic review of studies involving Chinese patients with AV disease (20 studies; 1,218 patients) found that the prevalence of BAV morphology was 10.9%16. In CARRY, 54% of patients with symptomatic severe AS undergoing TAVR screening had a BAV, with type 0 BAV being the most common (42.5% of BAV cases)22. In the SAPIEN 3 cohort, 50.9% of patients had a BAV, with type 1 BAV (29.0%) being slightly more common than type 0 (20.8%)23. This contrasts with a BAV rate of 17% that was reported for symptomatic AS patients in Europe24, among whom approximately 12% were type 02425. In addition to a potentially higher incidence of BAV-associated AV disease in the Chinese population26, possible reasons for the high prevalence of BAV among TAVR patients in China include treatment delay, disease awareness bias (patients with a BAV tend to be younger27 and more affordable to treat than older TV patients), and potential selection bias (e.g., centres in some countries may exclude BAV patients from TAVR)28.
Patients with a BAV enrolled in CARRY had a larger aortic annulus than those with a TV (mean 477.5 mm3 vs 451.2 mm3), a less elliptical annulus shape (mean eccentricity 21.7% vs 8.4%), and heavier calcification burden (558.7 mm3 vs 263.3 mm3)22. Similarly, in the SAPIEN 3 cohort, patients with a BAV had a larger mean annulus than patients with a TV (492.5±105.0 mm2 vs 456.9±85.8 mm2; p<0.001), and a greater percentage had severe aortic leaflet calcification (42.2% vs 27.9%)23. In addition, recipients of the SAPIEN 3 valve who had a BAV were younger than those with a TV (71.6±8.2 years vs 75.9±8.4 years).
Regional differences in AS characteristics have been noted within China. For example, in CARRY, the highest proportion of BAV cases was seen in Central China and the lowest in Northern China22. Patients from Northern China had the smallest anatomical features (e.g., annulus), whereas those from Western China had the most dilated ascending aorta. Patients in Central China had the highest calcification burden, and those in Eastern China had the lowest.
AS treatment rates in China
According to the Chinese Cardiac Surgery Registry (n=38,131), aortic valve surgery (for any indication) was performed in 50.2% of patients who underwent valve surgery procedures in China between 2016 and 201829, with the volume of isolated AV replacements increasing by 11.9% over this period30. The white book issued by the Chinese Society of Extracorporeal Circulation reported 77,077 cases of valvular surgery in 202131. Therefore, it can be estimated that there were approximately 38,000 SAVR procedures in 2021 (including both mechanical and bioprosthesis cases). Data from the NTCVR show that the number of TAVR procedures performed annually increased from 293 in 2017 to 7,357 in 202132.
TAVR technologies in China
The adoption of TAVR was slower in China than in Western countries33. Although the technology was first introduced in the country in 2010, devices only became commercially available from 2017 onwards20. Currently, four valves that were developed in China, as well as the non-domestic SAPIEN valves, are available commercially in the country. The Venus A-Valve (Venus Medtech) was the first to become available (2017) and is currently the most widely used in China33. This was followed by the J-Valve (JieCheng Medical; 2017), VitaFlow (MicroPort; 2019) and TaurusOne (Peijia Medical; 2021) devices34. The SAPIEN 3 device was approved in China in June 2020 and became available for use in September 2020.
The Venus A-Valve, J-Valve, VitaFlow and TaurusOne are self-expanding devices incorporating nitinol frames and trileaflet porcine3536 or bovine37 pericardial valves (Figure 1). The SAPIEN 3 valve is a balloon-expandable device incorporating a cobalt-chromium frame and trileaflet bovine pericardial valve (Figure 1)38. The SAPIEN 3, Venus A-Valve, VitaFlow and TaurusOne valves are implanted via the transfemoral route, whereas the J-Valve is inserted via the transapical route35363839.
Presently, there are more than 100 centres in China capable of performing TAVR33. According to the NTCVR, there were 83 active sites in 2021, an increase from 25 sites in 201740.
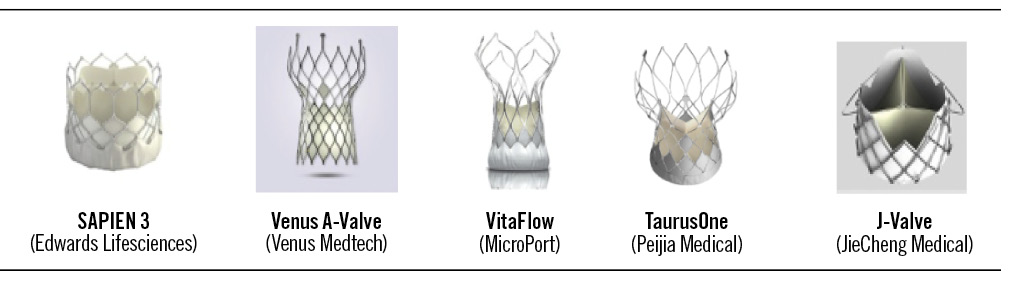
Figure 1. Transcatheter aortic valve replacement devices available in China12.
TAVR outcomes in China
Clinical outcomes for transfemoral TAVR in the Chinese population were evaluated using a systematic review methodology.
Search strategy
A search of global literature databases, including Medline (PubMed), Embase, and local Chinese databases (including CNKI, WANFANG MED, and VIP) was undertaken. An initial search was performed using the following terms (keywords): “TAVR”, “TAVI”, “transcatheter aortic valve replacement”, “humans”, “VitaFlow”, “SAPIEN 3”, “Venus-A”, “TaurusOne”, “clinical”, “death”, “mortality”, “second valve”, “valve-in-valve”, “PVL”, “paravalvular leak”, “PPI”, “PPM”, and “permanent pacemaker implantation”. Records published between 1 January 2009 and 17 May 2022 were included. In addition, summary data (data on file) were obtained for a cohort of patients who received a SAPIEN 3 valve during the first two years after it became available in China (June 2020-May 2022). Studies were excluded if they were not published in English or Chinese, did not have an abstract, were non-human studies, or were case studies.
Study selection
After the execution of the searches, duplicate records were removed, and the remaining set of unique studies were screened. Two independent reviewers examined eligibility for inclusion; a third reviewer acted as a tiebreaker for disagreements. Title and abstract screenings were performed, and those studies passing both screenings underwent a full-text evaluation to determine if they met the criteria for inclusion. To be included, studies must have enrolled only Chinese patients and reported separate clinical outcomes for one of the following TAVR valves: Venus A, VitaFlow, TaurusOne, or SAPIEN 3. In addition, studies must have reported one of the following clinical outcomes: mortality, need for new permanent pacemaker implantation (PPI), valve-in-valve, and paravalvular leakage rates.
Data extraction
Two reviewers extracted data from the studies included in the review. General study characteristics, including authorship, publication type, funding, and study setting, were recorded. Details related to study methodology, data sources, time horizon, and patient population were extracted, alongside the type of outcome(s) and results reported by the study.
Overview of studies
A total of 22 studies were identified323537394142434445464748495051525354555657, with 173235374142434447484950515253545556,
two3957, two2346 and one45 studies specific to Venus A-Valve, VitaFlow, SAPIEN 3, and TaurusOne, respectively. Among the 17 Venus A-Valve studies, 10 were Chinese-language studies47484950515253545556, and seven were English-language studies32353741424344. For VitaFlow, there was one Chinese-language57 and one English-language39 report. For SAPIEN 3, there was one Chinese46 and one English-language23 report. The single study identified for TaurusOne was in English45. Among the 22 studies, 13 studies were prospective, and the other nine were retrospective323537394142434445464748495051525354555657. Studies involved elderly patients (mean age 75.8 years old), and participants were equally distributed between males and females. Half (n=11) of the studies enrolled more than 100 patients2332373941434550545557, while four studies involved 50-99 patients42464956, five studies involved 20-49 patients3544475153, and two involved fewer than 20 patients4852.
Mortality
Thirteen studies reported 30-day all-cause and/or cardiovascular mortality rates23353941424346474851525556 (Table 1). Overall, the lowest 30-day all-cause mortality rate (0%) was reported in one of the SAPIEN 3 studies46, with the two other SAPIEN 3 studies both reporting a rate of 3.2%23. Among patients who received a VitaFlow valve, the 30-day all-cause and cardiovascular mortality rates were 0.9% and 1.8%, respectively57. In this study, all-cause mortality was higher in TV patients than in BAV patients (9.72% vs 7.42%). Among recipients of the Venus A-Valve, 30-day all-cause and cardiovascular mortality rates ranged from 3.7% to 10%3551 and from 4.5% to 10%4151, respectively. Two Venus A-Valve studies compared outcomes for patients with TV versus those with BAV, with neither finding a statistically significant difference in 30-day all-cause mortality between the two groups (Song et al: 6.8% in those with BAV vs 3.8% in those with TV; p=0.5043; Fu et al: 4.5% vs 5.4%41). Thirty-day mortality rates were not reported for TaurusOne.
Eight studies reported 1-year all-cause and/or cardiovascular mortality rates (Table 2)3739414554555657. The 1-year all-cause mortality and cardiovascular mortality rates reported for VitaFlow were 2.7% and 1.8%, despite mean Society of Thoracic Surgeons (STS) scores of around 8.8%, suggesting a high surgical risk population39. On the other hand, Yang et al reported a 1-year mortality for VitaFlow of 4.5%14. Among VitaFlow recipients, 1-year all-cause and cardiovascular mortality rates were numerically higher for TV patients compared to BAV patients (4.4% vs 0% and 2.9% vs 0%, respectively); however, it should be noted that TV patients were older (mean 78.55±4.76 years vs 76.41±4.56 years; p=0.0223) and had a higher mean STS score (9.72±6.28% vs 7.42±3.87%; p=0.019)39. Overall, among recipients of the Venus A-Valve, 1-year all-cause and cardiovascular mortality rates range from 5.9-13.6% and 11.4-12.2%, respectively. However, 1-year all-cause mortality did not differ significantly between TV and BAV patients who received the Venus A-Valve (13.5% vs 13.6%)41. The 1-year all-cause mortality rate for TaurusOne was 6.7%45. Among recipients of the SAPIEN 3 valve, 1-year all-cause mortality and cardiovascular mortality rates were 6.2% and 4.9%, respectively23.
Table 1. Thirty-day outcomes of the studies included in our systematic review of transfemoral TAVR in the Chinese population.
| Study characteristics | Outcomes, % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Year | Valve | N | Average STS | 30-day mortality | 30-day PVL | 30-day PPI | ViV | Majorvascularcomplications | |||
| All-cause | Cardio-vascular | Mild | Moderate | Severe | ||||||||
| Venus A | ||||||||||||
| Song et al43 | 2018 | BAV | 44 | 5.0 | 6.8 | 20.5 | 20.5 | 4.5 | ||||
| TV | 53 | 6.2 | 3.8 | 18.9 | 7.5 | 7.5 | ||||||
| BAV+TV | 101 | 5.5 | 5.3 | 18.8 | 15.8 | 5.9 | ||||||
| Fu et al41 | 2020 | BAV | 44 | 7.63 | 4.5 | 4.5 | 6.8 | 6.8 | 9.1 | |||
| TV | 74 | 6.70 | 5.4 | 5.4 | 9.5 | 1.4 | 2.7 | |||||
| Liao et al35 | 2017 | BAV+TV | 27 | 6.2 | 3.7 | 7.4 | 14.8 | |||||
| Li et al42 | 2020 | BAV+TV | 63 | >4 | 4.8 | |||||||
| Li et al [51 | 2018 | Unspecified | 30 | 2.4 | 3.3 | 3.3 | 10 | |||||
| Song et al37 | 2017 | Unspecified | 101 | 5.5 | 29.8 | 11.7 | 0 | 12.87 | 5.9 | |||
| Li et al51 | 2017 | Unspecified | 10 | 5 | 10 | 10 | ||||||
| Wang et al55 | 2019 | Unspecified | 238 | 7.14 | 5.9 | 17.6 | 10.5 | |||||
| He et al18 | 2021 | Unspecified | 129 | 16.1 | ||||||||
| Zhang et al44 | 2021 | Unspecified | 81 | 8.94 | 6 | 5.26 | ||||||
| Zhou et al48 | 2022 | Unspecified | 12 | 15.4 | 8.3 | 0 | 0 | |||||
| Zhou et al47 | 2018 | Unspecified | 25 | 4 | ||||||||
| VitaFlow | ||||||||||||
| Zhou et al39 | 2019 | BAV | 42 | 7.42 | ||||||||
| TV | 68 | 9.72 | ||||||||||
| BAV+TV | 110 | 8.84 | 0.9 | 1.8 | 0 | 2 | 0 | 2.7 | ||||
| SAPIEN 3 | ||||||||||||
| Pan et al46 | 2022 | Unspecified | 50 | 6.0 | 0 | 0 | 0 | 2.0 | ||||
| Edwards Lifesciences (Edwards Lifesciences, data on file, 2023)23 | 2023 | BAV+TV | 438 | 3.2 | 0.2 | |||||||
| Shang et al23 | 2023 | BAV+TV | 438 | 8.47 | 3.2 | 0.2 | ||||||
| BAV | 0.4 | |||||||||||
| TV | 0 | |||||||||||
| BAV: bicuspid aortic valve; PPI: permanent pacemaker implantation; PVL: paravalvular leakage; STS: Society of Thoracic Surgeons; TAVR: transcatheter aortic valve replacement; TV: tricuspid aortic valve; ViV: valve-in-valve | ||||||||||||
Table 2. One-year outcomes of the studies included in our systematic review of transfemoral TAVR in the Chinese population.
| Study characteristics | Outcomes, % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Year | Valve | N | STS | 1-year mortality | 1-year PVL | 1-year PPI | |||
| All-cause | Cardiovascular | Mild | Moderate | Severe | ||||||
| Venus A | ||||||||||
| Fu et al41 | 2020 | BAV | 44 | 7.63 | 13.6 | 11.4 | 9.1 | |||
| TV | 74 | 6.70 | 13.5 | 12.2 | 13.5 | |||||
| Song et al43 | 2017 | Unspecified | 101 | 5.5 | 5.9 | |||||
| Wang et al55 | 2019 | Unspecified | 238 | 7.14 | 8.4 | |||||
| Zhang et al44 | 2021 | Unspecified | 81 | 8.94 | 6 | 2.64 | 2.64 | |||
| VitaFlow | ||||||||||
| Zhou et al39 | 2019 | BAV | 42 | 7.42 | 0 | 0 | 0 | 0 | 14.3 | |
| TV | 68 | 9.72 | 4.4 | 2.9 | 0 | 0 | 22.1 | |||
| BAV+TV | 110 | 8.84 | 2.7 | 1.8 | 29 | 19.1 | ||||
| Yang et al14 | 2020 | Unspecified | 110 | 8.8 | 4.5 | |||||
| TaurusOne | ||||||||||
| Wang et al45 | 2022 | BAV+TV | 120 | 6.7 | 22.1 | |||||
| SAPIEN 3 | ||||||||||
| Shang et al23 | 2023 | BAV+TV | 225 | 8.47 | 6.2 | 4.9 | 6.7 | 1.3 | 4.4 | |
| BAV: bicuspid aortic valve; PPI: permanent pacemaker implantation; PVL: paravalvular leakage; STS: Society of Thoracic Surgeons; TAVR: transcatheter aortic valve replacement; TV: tricuspid aortic valve | ||||||||||
Pacemaker implantation
The need for PPI is one of the most common complications following TAVR, with an overall incidence of approximately 15% (about 25% with self-expanding valves and 7% with balloon-expandable valves)58. In this systematic review, three234155, six353943464852, and three394852 studies reported that patients underwent PPI during TAVR, within 30 days after TAVR, or ≥1 year after TAVR, respectively. Eight other studies reported PPI as an outcome but did not specify when PPI was received4249505152535657. Thirty-day and 1-year PPI rates are summarised in Table 1 and Table 2, respectively.
Among all Venus A-Valve studies that reported 30-day PPI rates for mixed TV/BAV cohorts, the lowest PPI rate was 7.4%, and the highest was 18.8%3543. In the study that reported an overall rate of 18.8%, the rate of PPI in BAV patients was 20.5% and 18.9% in TV patients43. The study that reported the lower rate of PPI (7.4%) had a very small sample size (n=27)35. The STS score is a predictive factor for long-term prognosis after a successful TAVR procedure59. Among the Venus A-Valve studies included in our systematic review, the highest and the lowest average STS scores were 15.4% and 2.4%, respectively4851. The likelihood of PPI after TAVR was found to be similar for BAV and TV populations after Venus A-Valve implantation41 and after SAPIEN 3 implantation23. Zhang et al 2021 reported that among patients who underwent PPI within 30 days after TAVR, the mean time to PPI following the TAVR procedure was 5.8±2.8 days44.
Among studies that reported 1-year PPI rates, the rates for Venus A-Valve (BAV 9.1% and TV 13.5%) were substantially lower than the rates reported for VitaFlow (BAV 14.3% and TV 22.1%), and TaurusOne (BAV/TV combined 22.1%)394145. One study reported a 2-year PPI rate of 19.10% with VitaFlow (cohort not specified as TV or BAV)43.
Second valve
Seven studies involving the Venus A-Valve35374143505155 and one involving the SAPIEN 3 valve23 assessed the need for a second valve implantation (Table 1). The rate was 0.2% with the SAPIEN 3 valve and ranged from 10.0% to 16.1% with the Venus A-Valve in cohorts that included both BAV and TV patients (or where the status was not specified). One study involving both BAV and TV patients, in which 15.8% of Venus A-Valve recipients required a second transcatheter aortic valve (TAV)-in-TAV during the procedure43, noted that oversizing of ≤15% was a contributing factor for TAV-in-TAV (p<0.001), with a rate of TAV-in-TAV of 26.2%, compared with 3.6% in patients with device oversizing >15% (p<0.001)43. In addition, BAV was associated with a higher TAV-in-TAV rate compared with TV in this study (20.5% vs 7.5%; p=0.06); however, this may be attributable to a significant difference in the degree of oversizing (BAV 13% vs TV 19%; p=0.001), as the frequency of oversizing ≥15% was 36.4% with BAV versus 73.6% with TV (p<0.001)43. Another Venus A-Valve study found no significant difference between TV and BAV patients in the rate of implantation of two prostheses during the TAVR procedure (1.4% vs 6.8%; p=0.145)41; this was also the case in the SAPIEN 3 study (0% vs 0.5%; p=0.491)23.
Paravalvular leak
Paravalvular regurgitation can be associated with death following TAVR, even if the aortic regurgitation (AR) is mild6061. In this systematic review, five studies examined rates of procedural AR2335415253, and seven studies evaluated 30-day rates39434648515256 (Table 1).
For the Venus A-Valve, one study observed that 11.5% of patients had more-than-mild AR during hospitalisation post-TAVR35. Two studies reported that rates of moderate/severe periprocedural paravalvular regurgitation were higher in BAV patients compared with TV patients (11.4% vs 6.0%43 and 11.4% vs 6.8%41), but the latter study indicated that the difference was not significant41. At 30 days after Venus A-Valve implantation, moderate and severe paravalvular regurgitation were reported in 0-11.7% and 0% of patients, respectively. One study reported that moderate paravalvular regurgitation occurred in 2.64% of patients at one year.
For VitaFlow, 2% of patients had moderate paravalvular leakage at discharge and after 30 days; none had severe leakage39. At 1-year follow-up, no patients had moderate or severe leakage. These low rates of moderate to severe paravalvular leakage might have contributed to the low all-cause mortality rates at discharge (0.6%) and at 30 days (0.9%)39.
In one of the SAPIEN 3 studies, no patient experienced moderate or worse paravalvular leakage up to 30 days46, while in the other study (which did not specify the time period), 0.5% of recipients had moderate paravalvular leakage, and 8.9% had mild leakage23. In the latter study, the rate of moderate paravalvular leakage was similar in patients with BAV or TV (0.4% vs 0.5%).
Major vascular complications
A study of the Venus A-Valve revealed a 5.9% rate of major vascular complications following TAVR, with 4.5% in BAV patients and 7.5% in TV patients, which is not statistically significant (p=0.54)43. Fu et al showed a higher incidence of major vascular complications at 30 days after TAVR in BAV patients (9.1%) than in TV patients (2.7%); p=0.19441. Another study reported a major vascular complication rate of the VitaFlow valve as 2.7%, with no significant difference between BAV and TV patients39.
The most recent SAPIEN 3 study revealed low (0.2%) periprocedural major vascular complications with no significant difference between BAV (0.4%) and TV (0%)23, while Pan et al reported a 2.0% rate of major vascular events in mixed BAV/TV cohorts46.
Summary
Although many studies had small sample sizes, the available data suggest that TAVR can be performed successfully in Chinese patients with AS using either self-expanding or balloon-expandable valves. The Venus A-Valve had the most data available, including evidence that there was generally no significant difference in outcomes between patients with BAV or TV. Direct comparisons between specific valve types in Chinese patients are lacking, and additional large studies are needed. Indirect comparison suggests that the Venus A-Valve might be associated with a lower rate of PPI compared with the VitaFlow or TaurusOne devices. Finally, the low 30-day mortality rate reported for SAPIEN 3 is consistent with the PARTNER 3 trial in Western patients62, suggesting that the efficacy of this valve extends to the Chinese population.
Future perspective on TAVR in China
It can be assumed that the demand for TAVR in China will continue to increase in the future due to the ageing population. Currently, around 100 hospitals in China offer TAVR, with most located in socioeconomically advanced conurbations with high-quality medical resources4041. It is anticipated that additional centres will develop expertise with this procedure in the coming years.
Aortic valve replacement must be suitable for the anatomical profile associated with Chinese AS patients, which includes a high burden of AV calcification and high prevalence of bicuspid pathology. With careful selection of patients and preprocedural assessment, similar rates of successful implantation can be achieved in patients with BAV or TV63. Currently, most TAVR devices used in China are self-expanding, with the Venus A-Valve being the most commonly used device. A balloon-expandable device (SAPIEN 3) was introduced to the Chinese market relatively recently. Limited evidence suggests similar overall safety and effectiveness may be achieved with self-expanding or balloon-expandable valves in Chinese patients. However, larger studies with extended follow-up are needed to confirm the relative benefits and risks of specific valves.
At present, SAVR remains the most common treatment for severe AS in China. The China-DVD Study found that 29% of patients with valvular heart disease refused surgery due to concerns about complications, cultural reservations about open-heart surgery, or affordability20. It is possible that TAVR may be more acceptable to some patients, as it is a less invasive procedure. Measures to educate patients about the benefits of interventions could increase treatment uptake in the future63. However, although health insurance has increased amongst the Chinese population in recent years, patients must still cover some of the costs of medical care64, and accessibility to and affordability of healthcare services remain problematic for elderly people65.
Limitations
Our review did not present data on patient-prosthesis mismatch due to a lack of information in the available literature selected for analysis.
Conclusions
AS represents a substantial healthcare burden in China, and this is likely to increase in the future due to the ageing population. Chinese AS patients have a high burden of AV calcification and high prevalence of bicuspid pathology. SAVR is currently the most common therapeutic strategy, but the use of TAVR is increasing. Direct comparisons between specific TAVR devices in Chinese patients are lacking, and additional studies are needed. Indirect comparison suggests a lower rate of PPI with the Venus A-Valve compared with the VitaFlow or TaurusOne devices. The low 30-day mortality rate reported for SAPIEN 3 suggests that the efficacy of this valve extends to the Chinese population.
Funding
This systematic literature review was funded by Edwards Lifesciences.
Conflict of interest statement
IPPMed Cloppenburg, represented by P. Bramlage, has received honoraria (or research funding) for consultancy from Edwards Lifesciences. The other authors have no conflicts of interest to declare.

