Introduction
Cardiovascular diseases (CVD) are a leading cause of death in Asia, as 58% of global CVD-related deaths occur in this region. Challenges related to the prevention and management of CVD in Asia have been mainly attributed to the diversity of ethnicities, socioeconomic factors, cultural practices, and healthcare systems in this region1. Additionally, the prevalence of CVD risk factors, such as smoking, unhealthy lifestyle practices, genetics, obesity, and the presence of chronic disorders (diabetes, hypertension), are also considerably high in Asian countries2. The disparity in access to healthcare resources is also a vital challenge in developing countries. Suboptimal treatment and undertreatment of CVDs are additional aspects that need to be considered2. Nevertheless, major advancements have been observed in CVD management with the increased availability of percutaneous coronary intervention (PCI) across the Asia-Pacific (APAC) region. According to 2016 estimates, more than one million PCI procedures have been conducted in the APAC region3, and this number continues to grow at a healthy compound annual growth rate (CAGR).
Research in health sciences drives innovation, empowers scientific leadership, and builds the economy4. Large volumes of PCI procedures and improved access to healthcare provide a unique opportunity for researchers to translate clinical practice into meaningful research4. However, there exists a wide variance in clinical pathways and protocols in different parts of the APAC region and, accordingly, a variation in how research is conducted.
According to global reports, the number of cardiovascular trials being conducted in the APAC region has increased by 38% over the past decade5. In 2020, the majority of trials were conducted in China (60%), South Korea (8%), and India (7%)5. However, guidance for researchers in cardiology is still lacking, which affects the optimal implementation of research methodologies to ensure effective outcomes6. Furthermore, there is a paucity of studies on the variability in the research environment in catheterisation laboratories (cath labs) across the APAC region. This study discusses the variability in research culture across busy cath labs in the APAC region. It also aims to produce a roadmap for hospitals to develop in-house research capabilities in conjunction with the healthcare industry and academia.
Methods
This study was a combination of primary and secondary searches and was conducted in 2 stages. In stage 1, between 1 April 2022 and 30 April 2022, an online survey questionnaire was sent to more than 75 cath labs/hospitals undertaking at least 250 angioplasty procedures annually across different APAC countries, including Australia, China, Hong Kong, India, Indonesia, Japan, Malaysia, New Zealand, the Philippines, Singapore, South Korea, Taiwan, and Thailand. The selection of participating labs was based on the following considerations: (a) case volume (more than 250 PCI/year); (b) clinical research experience (verified participation in at least one study listed in trial registries); (c) recommendations from healthcare industry sources on hospitals that have shown interest in research activities; (d) university affiliation (at least 10% of participating cath labs were chosen from among university-affiliated hospitals); (e) distribution across geographies (in proportion to PCI volume). Independent core labs were excluded. The survey comprised questions related to the status of research activities undertaken by these cath labs, infrastructure (including workforce), number of ongoing studies (clinical/non-clinical), budget allocation, publications, management review, and patents. The survey questions are presented in Supplementary Appendix 1. The results obtained were compiled and analysed for trends and variabilities seen across these countries. The survey responses were kept anonymous to maintain confidentiality and eliminate bias in stage 2 of the study.
In stage 2, the survey results were shared with a team of 10 leading cardiology researchers from Australia, China, Thailand, India, Japan, South Korea, and Singapore (each with a minimum of 50 publications/presentations) to understand the trends in clinical research and identify the challenges in setting up institutional research capabilities in the APAC region. A roadmap to achieve the same was then suggested to support these cath labs/hospitals develop their research capabilities (Central illustration).
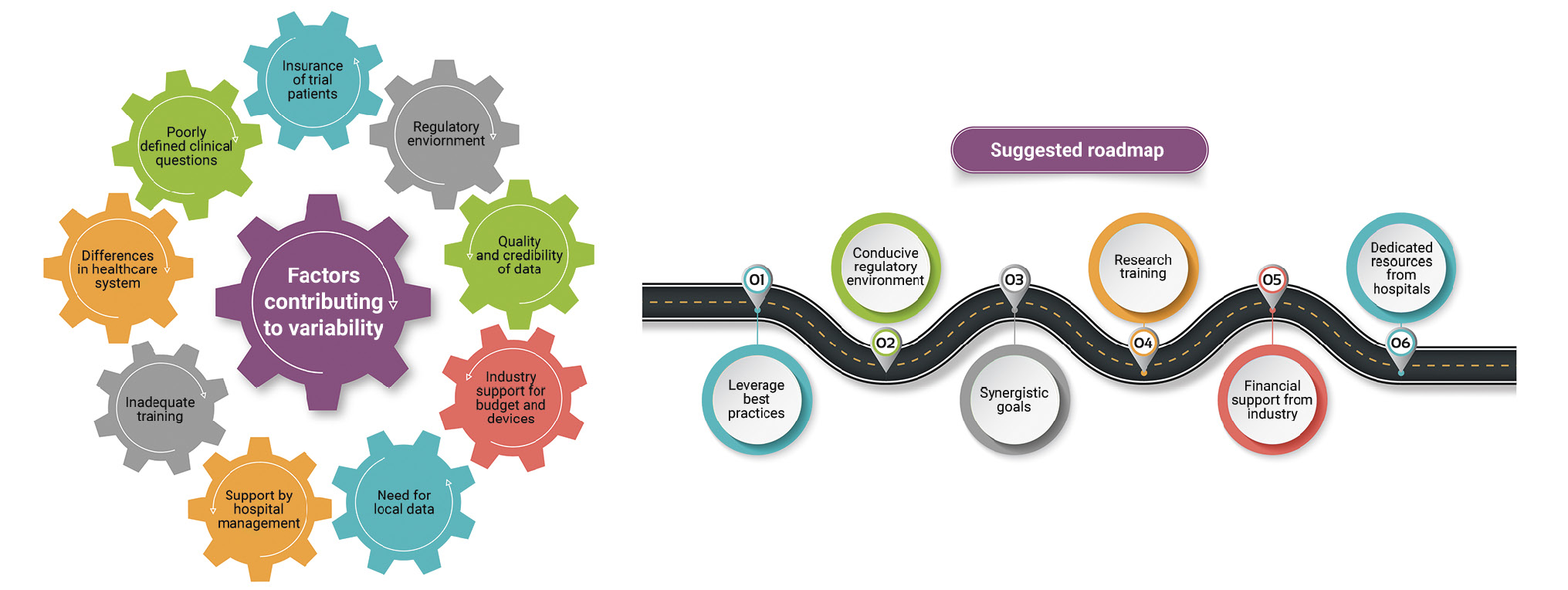
Central illustration. Illustration of factors contributing to variability and proposed roadmap. A survey was conducted to assess the variability in research culture in busy catheterisation labs across the Asia-Pacific (APAC) region. The survey outcomes were examined by a group of cardiology experts. The expert discussion resulted in the identification of factors contributing to variability. A roadmap was also proposed for supporting research in APAC settings.
Results
Survey results
Demographics
Survey responses were received from a total of 220 respondents from 62 cath labs (88.6%) from 13 APAC countries, including Australia, China, Hong Kong, India, Indonesia, Japan, Malaysia, New Zealand, the Philippines, Singapore, South Korea, Taiwan, and Thailand. The respondents in these countries comprised cath lab directors, heads of cardiology departments, heads of research, senior interventional cardiologists, fellows, physician assistants, and clinical research coordinators. The number of respondents was evenly distributed across countries to eliminate regional bias except for smaller countries such as Indonesia, the Philippines, and Vietnam. The respondents were from both public and private institutions.
Types of studies conducted
Figure 1 provides an overview of the types of studies conducted in the enrolled cath labs. The majority of the studies conducted were prospective (147, 67%); they were primarily multicentre (178, 81%) and non-randomised (194, 88%) studies and involved a single country (196, 89%). A large proportion of these studies were investigator initiated (147, 67%). Furthermore, the majority of studies were placebo-controlled (or sham-controlled) for evaluating medications, whereas only a few studies involved devices – these accounted for less than 5% (n=11) of the total research volume.
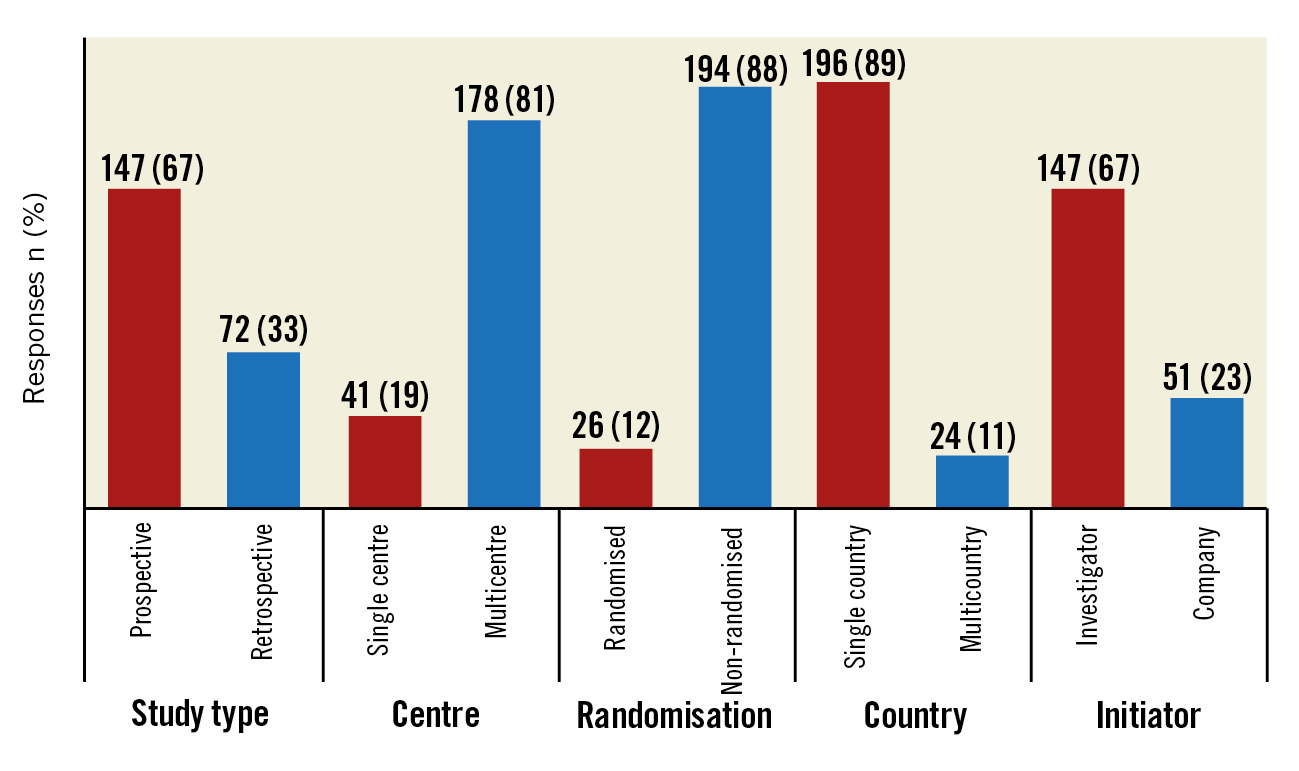
Figure 1. Types of studies conducted.
Clinical research infrastructure
A large majority of the cath labs had dedicated clinical research units (125, 57%) with annual Good Clinical Practice/International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (GCP/ICH) certification (191, 87%) (Figure 2). Moreover, 29% (n=64) of the cath labs had regulatory/sponsor audit experience. Patient screening rooms and record archival facilities were available in 21% (n=46) and 28% (n=62) of the cath labs, respectively. The availability of an in-house biostatistician or tie-up with a university (33, 15%), medical writing capabilities (42, 19%), and the ability to conduct pharmacokinetic studies (20, 9%) were limited.
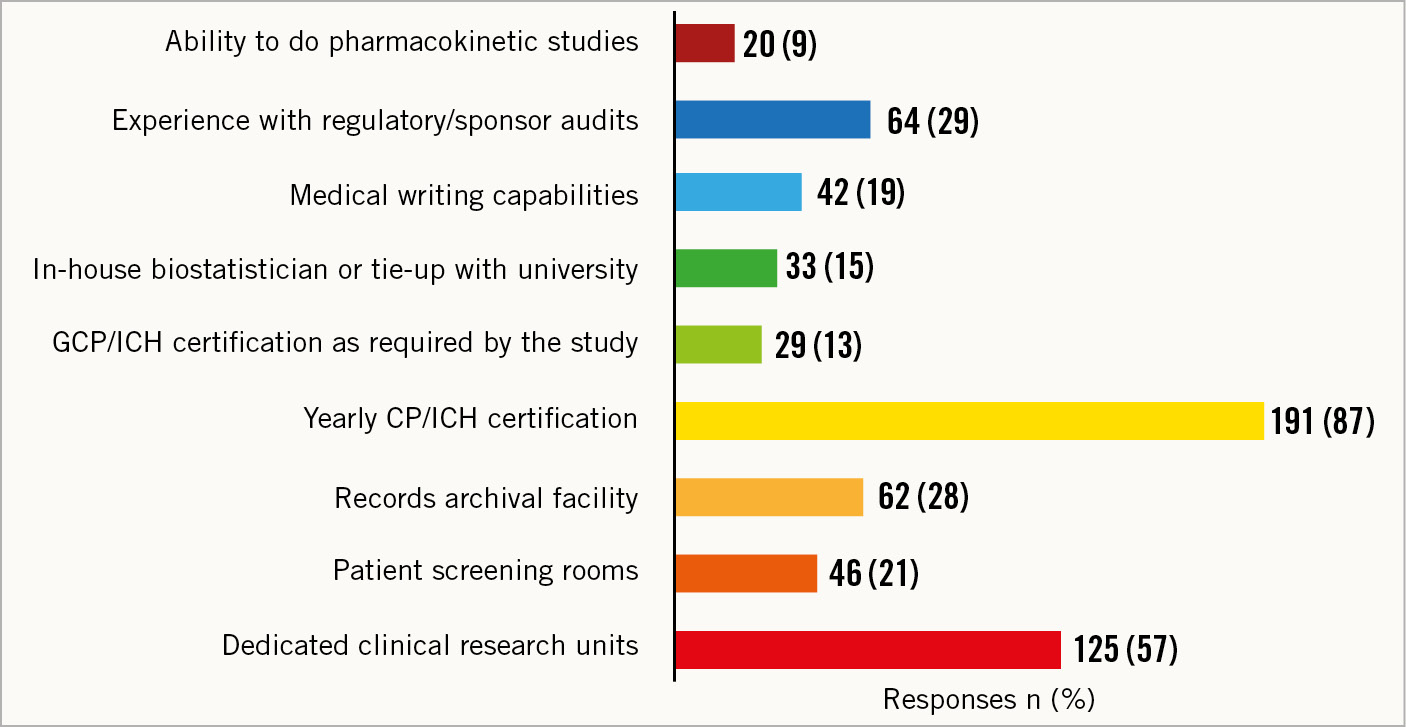
Figure 2. Clinical research infrastructure. GCP/ICH: Good Clinical Practice/International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use
Number of peer-reviewed publications/presentations in national/international conferences
A majority of the respondents reported publishing <10 peer-reviewed publications/presentations (90, 41%), whereas 34% (n=75) reported publishing/presenting 10-25 articles per year (Figure 3). Notably, approximately 2% (n=4) of the respondents reported publishing >100 articles per year.
However, none of the respondents were the lead principal investigators in 76% (n=167) of cases, whereas 4% (n=9) of the respondents (from Australia, Japan, and South Korea) were the lead investigators for >10 studies (Table 1).
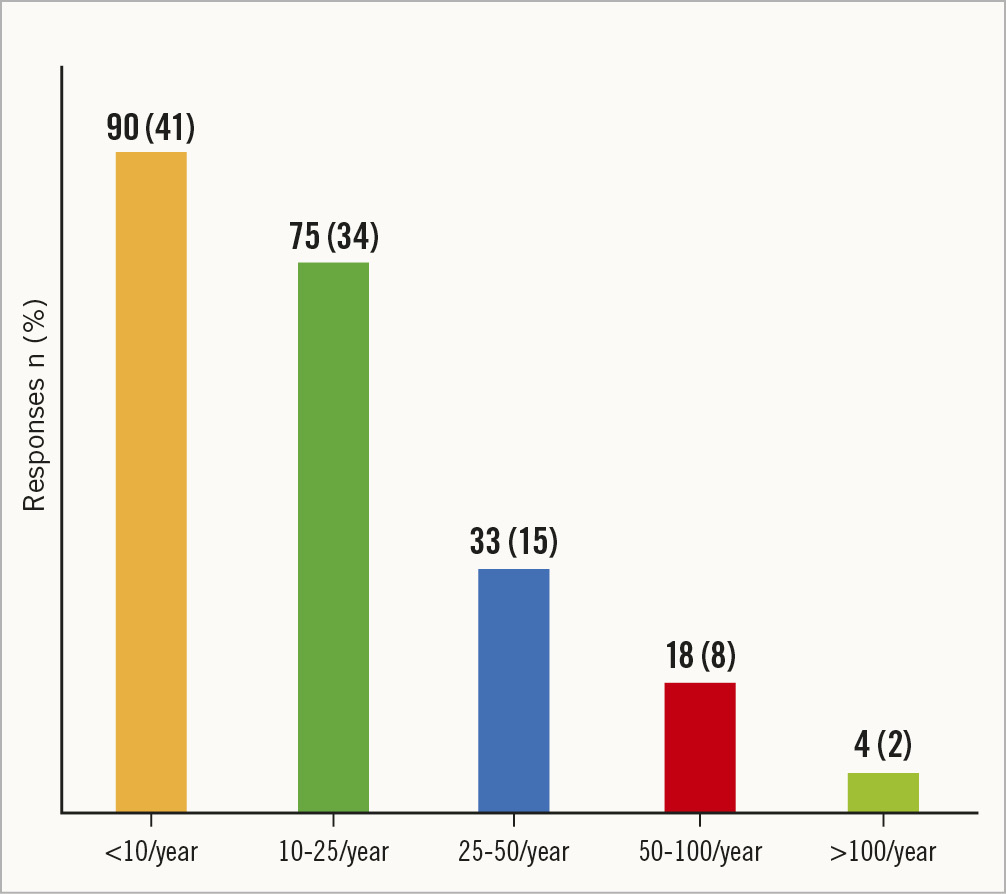
Figure 3. Number of peer-reviewed publications/presentations in national/international conferences per year.
Table 1. Percentage of respondents who are/were lead investigators in the studies conducted.
| Number of studies as lead principal investigators | Count (%) |
|---|---|
| None | 167 (76) |
| 1-5 | 18 (8) |
| 6-10 | 26 (12) |
| >10 | 9 (4) |
Funding sources
Most of the studies conducted at the enrolled centres were funded by the healthcare industry (189, 86%), followed by government grants (15, 7%), universities (11, 5%), professional societies (2, 1%), and research charities (2, 1%).
Review results
The survey findings were reviewed by 10 leading researchers in the field of cardiology to understand the gaps in knowledge and develop a roadmap for supporting research in the APAC region. According to these researchers, the factors contributing to the variability in research activities in the APAC region included the following:
- Differences in healthcare systems across these countries, which result in variations in the delivery of healthcare;
- Regulatory environment and ease of performing trials; supporting, or even discouraging the testing of new devices;
- Support by hospital management, including providing dedicated resources, space, and technology for research activities;
- Need for local data before and after approval of new devices;
- Quality and credibility of data;
- Healthcare industry support for budget and devices;
- Inadequate training;
- Poorly defined clinical questions;
- Insurance of trial patients.
The experts also opined that an ideal research cath lab should have the following:
- Dedicated clinical research unit with experienced staff and equipment;
- ICH Guideline for GCP certification;
- Periodic regulatory/sponsor audits;
- Patient screening facility;
- Record archival facility.
The experts then suggested a roadmap to develop and sustain the research culture in busy cath labs in the APAC region (Figure 4). The major building blocks of this roadmap include the following elements:
- Leverage best practices: new institutions do not have to reinvent the wheel and should follow the best practices of established leaders;
- Conducive regulatory environment: research staff should be aware of the regulatory requirements to conduct studies efficiently and increase the chances of acceptance/publication of the results. Regulatory bodies should simplify the research requirements related to the research being conducted at cath labs;
- Synergistic goals: effective collaborations to identify mutual synergistic goals; investigators should identify and document key performance metrics for their research projects and measure them against the outcome; such metrics could be the budget, publication, presentation, or even a patent;
- Research training: periodic clinical research and medical writing training/workshops should be provided for physicians and research staff to acclimatise them to new developments; identifying the roles and responsibilities of principal investigators, support staff, and sponsors so that each of them can optimise the study process is also needed;
- Industrial financial support: adequate financial support from the healthcare/pharmaceutical industry is essential and can help increase the research activities being conducted;
- Dedicated resources from hospitals: hospitals that encourage research should provide adequate research infrastructure in terms of staff, equipment, and other related aspects.
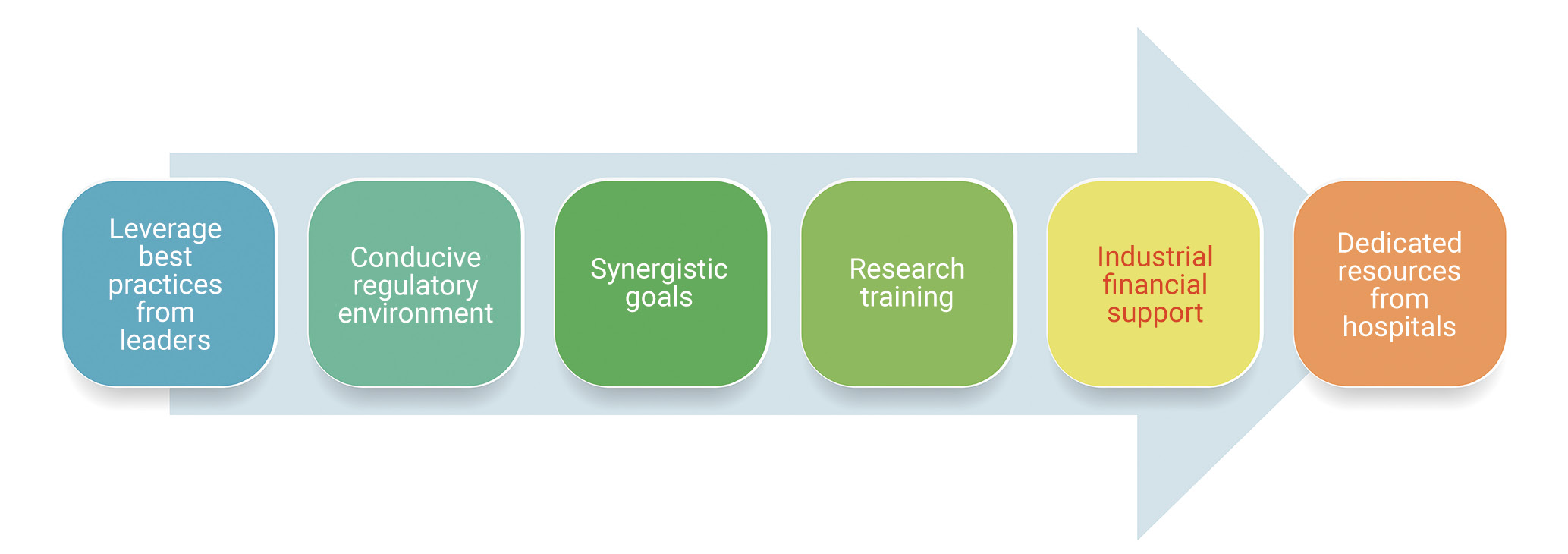
Figure 4. Roadmap to bridge gaps in research culture in cath labs in the APAC region.
Discussion
The progressive evolution of cardiac catheterisation procedures over the last few decades has been supported by numerous evidence-based trials conducted globally, which have been adequately reflected in the guidelines published by several organisations. These developments have led to an increased uptake of catheterisation procedures worldwide7. Although the technology is evolving, patient and family-centred outcomes remain the key focus of healthcare providers, funding agencies, and policy-forming organisations6. Therefore, conducting well-designed clinical trials to critically evaluate procedural outcomes is of utmost importance. Some of the aspects that play a vital role in determining the outcomes in clinical trials include well-designed protocols, robust site-based research infrastructure, and well-qualified site teams8. With the APAC region serving as a hotspot for clinical trials in different fields of medicine and technology, it is crucial for its research centres to gear up and address the challenges of conducting extensive research.
In the last two decades, the APAC region has been home to a few “first-in-human” trials for some of the leading drug-eluting stent (DES) technologies, although such trials were limited to a handful of countries and were driven by the prevailing regulatory environment910111213. Several post-marketing studies involving DES technologies were also conducted in the APAC region, which subsequently supported the generation of additional evidence outside the scope of approval trials. Many landmark trials involving novel concepts, like PCI in the left main, PCI versus coronary artery bypass graft (CABG), and PCI of long lesions were also conducted as part of investigator-initiated studies in some APAC countries1415. The Short and Optimal Duration of Dual Antiplatelet Therapy (STOPDAPT) trial series from Japan significantly contributed to the global evidence on the optimal dual antiplatelet therapy (DAPT) regimen after PCI16. Most of these trials were presented as “late breakers” in leading international conferences and published in leading medical journals (Table 2).
The results of our study indicate a wide variation in both the quality and quantity of the research conducted across different APAC countries. There was a very limited appetite for preclinical research or bench testing of devices. Only a few cath labs in Australia, Japan, New Zealand, and Singapore were technically equipped to undertake this type of research. Such research projects were often conducted with the help of local universities which had the required infrastructure. The area of interest was usually a comparison of different commercially available products that were often tested against prototypes.
It was also noted in our survey that countries such as Australia, New Zealand and South Korea have well-established research set-ups for conducting all types of research activities, including bench testing, as compared to other countries such as India, China, Indonesia, and the Philippines, where research facilities are more limited. Notably, retrospective studies were preferred in China due to the large patient volume. There is huge research potential in countries such as China and India to organise large-scale studies, given the large patient volumes and adequately trained workforce available in these countries. Our survey also noted that large multicentre trials are very common in South Korea, with various physicians’ groups joining hands to conduct such studies. Furthermore, real-world registries are more common than randomised control trials in the APAC region.
It was evident from our survey that routine follow-up of patients is well established in Japan with well-maintained medical records. However, linking medical records across different hospitals/states was considered challenging in most APAC countries, except Australia and New Zealand. Nevertheless, any form of data linkage may involve a significant amount of time and resources, even in countries such as Australia and New Zealand. It was also observed in our survey that leading research hospitals usually collaborate with local universities and avail of their biostatistics and medical writing services; in addition, adequate support is provided by the heads of the departments and senior consultants to research fellows to organise hospital-level research and publish their work. However, this was limited to less than 20% of the surveyed cath labs.
Healthcare industry funding was noted in 49.9% of the clinical trials conducted in the APAC region in 2020; moreover, the percentage of clinical trials funded by the healthcare industry increased by 43% over the previous decade17. According to the results obtained from this survey, 86% of the respondents mentioned that the clinical trials being conducted at their organisations were funded by the healthcare industry. As partnering among pharmaceutical organisations, biotechnology companies, and research institutions is on the rise, steps need to be taken to improve regulatory requirements and institutional policies for streamlining research and improving outcomes18. According to an article on funded studies, pharmaceutical industry-sponsored trials evaluated innovative therapies, had stronger evidence, were associated with a high proportion of open-access articles (63%), and had an equal emphasis on negative and positive results19. As per unconfirmed healthcare industry sources, many medical technology companies have strengthened their academic partnerships through investigator-initiated studies. The healthcare industry is always looking for novel study ideas to bridge the gap between unmet clinical needs and evidence. Therefore, the healthcare industry has taken initiatives to identify new researchers and expand the research footprint outside of the well-known and published key opinion leaders.
As noted in this survey, single-country studies were the most common clinical trials, accounting for 91.3% of all clinical trials in the APAC region. Traditional clinical trials are considered challenging because of their high costs, length, and complexity, in addition to their complex institutional and regulatory requirements20. According to a qualitative study from China, inadequate protection of research participants, lack of technical infrastructure, and lack of resources were the major challenges to conducting clinical trials in this region18. Nevertheless, the study also reported a positive trend in such trials with increased funding from the government, supportive policies, high industrial demand, and wider recognition for clinical research18. Limited time and lack of experience related to ethical regulations, clinical research design, methodology, and implementation, along with high expenditure, lack of peer support, and unfamiliarity with academic writing were the other challenges reported182122. Research training in several countries occurs ad hoc as healthcare professionals initiate clinical trials. Emphasis on education and training related to clinical trials during medical or nursing education is often lacking. This results in a large variation in the background and training needs of clinical investigators in the APAC region8.
The experts who were involved in this study suggested that the research culture in the APAC region can be improved through partnership, clearly identified roles and responsibilities, leveraging best practices, identifying key performance metrics, support from the management, and periodic clinical research and medical writing training/workshops for physicians and research staff.
The development of research training programs in association with research institutions should be planned. Training courses can be tailored according to the target audience, type of content, and study and should be offered to principal investigators, ethical committee members, and study coordinators18. Qualitative methods can also be followed for more complex research programs involving large amounts of data and requiring a comprehensive understanding of the issue being assessed6. Furthermore, offering protocol-specific training can also help the research staff better develop their skills through mentorship. Discussion of hypothetical scenarios or case studies, role-playing, and interactive sessions with peers are some of the additional ways through which research training can be improvised23. Site-based training activities, such as job shadowing, knowledge sharing (in-person or virtually), and mock visits or protocol procedures, can also help provide practical training to the individuals involved in clinical research8.
Clinical trials require significant cooperation among the different stakeholders, including clinical investigators, physicians, research institutions, sponsors (academia, healthcare industry, non-profit organisations, government), insurance companies, patients/caregivers, and regulators24. According to GlobalData, the majority (54%) of the clinical trials in 2022 were scheduled to be performed in the APAC region. Among all the countries in the APAC region, China was in the top position (53%) followed by India (17%) and Australia (12%) in conducting such studies25. There were about 199 clinical trials planned on CVD in the APAC region. Furthermore, the APAC region has been identified as the fastest-growing destination for medical device clinical trials, with China and Japan considered the top clinical trial hotspots25. The APAC region has also been positioned as the second largest market for medical devices26. Accordingly, it is essential to upgrade the capacity of the research organisations in the APAC region, to increase their independence and sustainability in clinical research. Investing in population-based registries and CVD surveillance is also vital for increasing the quality of clinical research as well as reducing the morbidity and mortality associated with CVD.
Table 2. Some landmark trials from the APAC region.
| Trial phase | Trial name | Trial type | Participating APAC countries |
|---|---|---|---|
| Pre-approval | SPIRIT II27 | Single-blind multicentre, non-inferiority randomised (3:1) controlled trial | Australia, New Zealand, India |
| ABSORB Cohort A&B28 | Prospective, open-label study | Australia, New Zealand | |
| ZoMaxx I29 | Randomised, controlled, multicentre trial | Australia, New Zealand | |
| MeRes-130 | First-in-human, single-arm, prospective, multicentre trial | India | |
| Regulatory approval trials | ABSORB China31 | Randomised multicentre trial | China |
| ABSORB Japan32 | Randomised controlled trial | Japan | |
| Post-approval trials | BEST33 | Prospective, multicentre, randomised controlled trial | South Korea, China, Malaysia, Thailand |
| Tuxedo34 | Randomised controlled trial | India | |
| IVUS-XPL35 | Retrospective and prospective follow-up study | South Korea | |
| STOPDAPT series (I, II & III)1636 | Multicentre, open-label, randomised controlled trial | Japan | |
| ULTIMATE37 | Multicentre randomised controlled trial | China | |
| DKCRUSH-V38 | Randomised controlled trial | China, Indonesia, Thailand, Italy |
Limitations
This study has some limitations that should be considered when interpreting the findings. The selection of cath labs/hospital for the survey was based on predefined criteria, which could potentially cause a selection bias. Due to this, labs with limited research activities may have been underrepresented, which limits the generalisability of these results across facilities in the broader APAC region. Additionally, as with all studies relying on self-reported data from surveys, the possibility of reporting bias should also be considered. Given the lack of independent verification or validation of the reported data on research activities, infrastructure, budgets, financial conflicts, and challenges faced by the respondents, these findings must be carefully interpreted.
Conclusions
There is a wide variance in how research is conducted in different APAC countries. It is prudent for physicians interested in setting up research projects to partner with well-developed research institutions and conduct collaborative projects to adopt best practices. Creating a repository of well-defined clinical data and risk-adjusted outcomes − which can be used to identify and close gaps in the quality of care, reduce variation and disparities in care, improve efficiency, and lower the cost of care − will be very useful.
Impact on daily practice
Research in the catheterisation laboratories in APAC region has grown significantly, especially with the availability of newer devices; China and India have huge research potential with regard to clinical trials in this area of medicine. Variations exist in the research infrastructure, quality of studies, and amount of training received by physicians and researchers across the APAC region. Research training programs should be conducted in the APAC region to increase the quality of research; involvement of the healthcare industry is crucial in improving the quality and quantity of clinical research at cath labs.
Acknowledgements
The work and contribution of the participating cardiology researchers who played a vital role as subject matter experts is greatly appreciated. Without their effort, this study would not be possible. We would like to thank the following interventional cardiologists: Dr Nigel Jepson (Eastern Heath Clinic, Australia), Dr Sidney Lo (Liverpool Hospital, Australia), Dr Adrian Low (National University Hospital, Singapore), Dr Nattawut Wongpraparut (Siriraj Hospital, Thailand), Dr Rony Mathew (Lisie Hospital, India), Dr Ajith Mullasari (MMM, India), Dr B.K. Koo (Samsung Hospital, South Korea), Dr J.M. Lee (Samsung Hospital, South Korea), Dr Takeshi Akasaka (Wakayama University, Japan), Dr Toshiro Shinke (Showa University Hospital, Japan), Dr Xu Bo (Fuwai Hospital, China), and Dr Haibo Jia (Second Affiliated Hospital of Harbin Medical University, China).
Conflict of interest statement
M. Narang, A. Saxena, R. Kaur and N.E.J. West are employees of Abbott Vascular. H. Reddy Gopa is an employee of BioQuest Solutions Pvt Ltd.
