Introduction
Percutaneous coronary intervention (PCI) with stent implantation using drug-eluting stents (DES) is the most widely performed intervention to treat coronary artery disease (CAD)1. However, poor PCI outcomes have been seen in diffuse, long coronary artery lesions. Such lesions have an increased risk of stent thrombosis (ST) and restenosis2. Stent size mismatch has been identified as an important factor in restenosis in long lesions. In this type of lesion, the coronary vessel diameter significantly changes over the length of the stent; such vessels are known as tapering arteries3. Tapering coronary arteries are common in clinical practice and often pose a dilemma in deciding the optimal stent size during PCI45. Studies have shown that the dimensions of coronary arteries taper naturally along their length. It has been reported that 23% of coronary arteries had a ≥1 mm taper and 19% had a 0.50-0.99 mm taper6. Long, complex and diffuse lesions in tapering arteries are treated by deploying either multiple stents or a single long stent. Multiple short, overlapping stents with variable diameters are often implanted to adequately match the size of long, tapered lesions1. However, stent overlapping is associated with higher neointimal proliferation. This increases the risk of restenosis due to higher vascular injury rates, delayed healing, very late ST, vessel aneurysm, or side branch jailing. Moreover, it increases the cost of treatment and leads to overuse of antirestenosis drugs and increased exposure to radiation and contrast media7. While stent oversizing can induce pathological stress on the arterial wall and lead to aneurysm formation, late ST, and even late perforations, stent undersizing can cause ST due to stent malapposition8.
A novel dedicated long, tapered DES may overcome these challenges of deploying stents in tapering coronary arteries. A tapered stent can provide a safer approach over overlapping stents9. Meril Life Sciences introduced the long and tapered BioMime Morph sirolimus-eluting coronary stent system (SES), without any changes in the core stent design of BioMime SES (Meril Life Sciences), for treating long coronary lesions. This long and tapered stent system is designed for de novo lesions with lengths >26 mm and ≤56 mm in native coronary arteries. Previous studies have reported the procedural success rates and 1-year safety and efficacy outcomes of this stent system for long coronary lesions5101112131415. This study was undertaken to evaluate the safety and performance of the aforementioned BioMime Morph SES in very long (length >26 mm and ≤56 mm) coronary lesions in native coronary arteries with reference vessel diameters of 2.25 mm to 3.50 mm.
Methods
Study design and patient population
Morph India was a single-arm, multicentre, observational, real-world, post-marketing surveillance study. The objective of the study was to evaluate the long-term safety and performance of the BioMime Morph SES for very long coronary lesions (length >26 mm to ≤56 mm) in native coronary arteries with reference vessel diameters of 2.25 mm to 3.50 mm. Nineteen sites participated in the study.
The patients were required to provide written informed consent prior to the study enrolment and adhere to the study schedule of events. The institutional review board or independent ethics committee at each clinical site approved the study protocol. The trial was registered in the Clinical Trials Registry – India (CTRI/2016/12/007527).
Study device
BioMime Morph is the world’s first commercialised, European Conformity-marked, long and tapered coronary SES, designed to precisely address the anatomical issues of vessel tapering and flexibility. The technical specifications and unique characteristics of the BioMime Morph SES have been reported previously116. The BioMime Morph SES is an L605 cobalt-chromium alloy balloon-expandable stent with a 65 μm ultrathin strut. It consists of a tapered, rapid-exchange stent delivery system, an active pharmaceutical ingredient – sirolimus − and biodegradable polymers – poly L-lactide and poly-D, L-lactide-co-glycolide. It is based on the well-regarded BioMime SES, which was indigenously designed and developed by Meril Life Sciences Pvt. Ltd., India. The stent is inflated with a tapered balloon, on which the stent system is mounted. The stent system is suitable for achieving proximal to distal tapering diameters of 2.75-2.25 mm, 3.0-2.5 mm, 3.5-2.75 mm, and 3.5-3.0 mm and is available in lengths of 30 mm, 40 mm, 50 mm, and 60 mm. The salient features of the device are presented in Supplementary Figure 1.
Eligibility criteria
The inclusion criteria were as follows: (1) age >18 years; and (2) significant native coronary artery stenosis (>50% by visual estimate) with a lesion length >26 mm to ≤56 mm. The exclusion criteria were the following: (1) a history of hypersensitivity reactions to aspirin, heparin, clopidogrel, cobalt-chromium, contrast agents, or sirolimus or a contraindication for any of these medications; (2) a planned elective surgical procedure that would necessitate the interruption of antiplatelet drugs during the first 6 months post-enrolment; and (3) active participation in another drug or device investigational study.
Baseline and procedural assessments
After obtaining written informed consent, the past medical records of subjects were examined. At baseline, patients underwent coronary angiography and assessments per routine clinical practice. PCI with DES implantation was performed according to the standard practices at each participating institution, in accordance with the ACC/AHA guidelines for myocardial revascularisation17. During the index procedure, anticoagulation was maintained by the infusion of unfractionated heparin. Following PCI, the patients were prescribed antiplatelet therapy (aspirin+ticagrelor/clopidogrel/prasugrel) per the investigator’s discretion, in accordance with the recommendations of the ACC/AHA or European Society of Cardiology (ESC) regarding optimal anticoagulant administration18. Electrocardiography was performed as required until the 1-year follow-up visit, and if necessary, at the 2-year and 3-year follow-up visits based on the clinical indication. The clinical follow-up visits were done at 1 and 6 months, and 1, 2, and 3 years.
Outcome measures
The primary endpoint was freedom from target lesion failure (TLF) throughout the study. TLF was defined as a composite of cardiac death attributed to the target vessel, myocardial infarction (MI) attributed to the target vessel (TVMI) or ischaemia-driven target lesion revascularisation (ID-TLR).
Other endpoints were the following:
- Target vessel failure (TVF): defined as a composite of cardiac death related to the target vessel, TVMI, and ischaemia-driven target vessel revascularisation (ID-TVR). If it could not be determined with certainty whether the MI or death was related to the target vessel, it was considered a TVF;
- Cardiac death due to immediate cardiac cause, death related to the procedure, unwitnessed death, and death from unknown cause;
- TVMI: all MI related to the target vessel. MI was defined per the third universal definition of myocardial infarction19;
- ID-TLR;
- Target vessel revascularisation (TVR): defined as a repeat percutaneous intervention or bypass surgery of the target vessel;
- Procedural success: defined as angiographic evidence of <30% final residual stenosis of the target lesion after stent placement and no occurrence of a procedure-related adverse event prior to hospital discharge;
- Device success: defined as angiographic evidence of <30% final residual stenosis of the target lesion using only the assigned device;
- Late lumen loss (LLL): defined as the difference between postprocedural minimum luminal diameter (MLD) and follow-up MLD at 9 months (±28 days) as assessed by quantitative coronary angiography (QCA). Approximately 10% of the target population was considered for LLL analysis.
All clinical safety events including TLF, TLR, TVF, TVMI, cardiac death, ID-TVR, and TVR were recorded at 1 month, 6 months, and at 1, 2, and 3 years. QCA data were utilised to assess the LLL, defined as the difference in MLD between the postprocedural and the 9-month angiographic follow-ups. ST was classified according to Academic Research Consortium definitions20.
Statistical analysis
The data of the enrolled population were analysed according to the intention-to-treat principle. Continuous variables are summarised as mean±standard deviation (SD), while categorical data are presented as frequencies with percentages. The safety analysis was carried out after excluding the dropout cases and subjects lost to follow-up. The evaluation of SES performance was conducted at the 9-month follow-up in a subset of eligible subjects by performing QCA analysis, in order to reduce the risk of contrast agent-related adverse events. A post hoc subgroup analysis was conducted to assess the efficacy of the device in the setting of very long coronary lesions among the patients who received 50 mm and 60 mm stents. The statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) version 21 (IBM).
Results
In all, 448 patients were enrolled across 19 centres. Overall, 420 subjects completed the 3-year follow-up, 23 patients were lost to follow-up, 3 withdrew consent (1 patient withdrew consent at 12-month follow-up after an event but was considered to be part of the study population for 3 years to calculate the cumulative event rates), 1 patient was terminated from the study by the investigators, and 2 patients were withdrawn by the investigators after the index procedure. The details of patient flow are provided in the CONSORT study diagram (Supplementary Figure 2).
Baseline characteristics and medical history
The enrolled study population (n=448) comprised 83.93% males, and the mean age of the patients was 58.00±10.22 years. The baseline characteristics are shown in Table 1 and the Central illustration. There was a high prevalence of risk factors such as diabetes mellitus (48.88%) and hypertension (48.66%).
Table 1. Baseline demographics and medical history of all patients.
| Characteristic | Total study population (n=448) |
|---|---|
| Age, years | 58.00±10.22 |
| Sex | |
| Male | 376 (83.93) |
| Female | 72 (16.07) |
| Smoker | 102 (22.77) |
| Current smoker | 60 (13.39) |
| Former smoker | 42 (9.38) |
| Alcoholic | 62 (13.84) |
| Current alcoholic | 30 (6.7) |
| Former alcoholic | 32 (7.14) |
| Diabetes | 219 (48.88) |
| Hypertension | 218 (48.66) |
| Hyperlipidaemia | 54 (12.05) |
| Renal insufficiency | 8 (1.79) |
| Previous MI | 58 (12.95) |
| Previous PCI | 38 (8.48) |
| Previous CABG | 9 (2.01) |
| Family history of CAD | 53 (11.83) |
| Stable angina | 86 (19.20) |
| Unstable angina | 154 (34.38) |
| STEMI | 144 (32.14) |
| Non-STEMI | 54 (12.05) |
| Silent ischaemia/asymptomatic | 10 (2.23) |
| Values are presented as n (%) or mean±SD. CABG: coronary artery bypass grafting; CAD: coronary artery disease; MI: myocardial infarction; PCI: percutaneous coronary intervention; SD: standard deviation; STEMI: ST-segment elevation myocardial infarction | |
Lesion and procedural characteristics
There was a total of 459 coronary lesions, of which 99.13% were de novo lesions (n=455) and 0.87% were in-stent restenosis (ISR) (n=4). Lesion characteristics are shown in Table 2 and the Central illustration. Approximately 57% of patients had Thrombolysis in Myocardial Infarction (TIMI) flow grade 0 or 1 before the procedure.
As shown in Table 2, the study device was successfully implanted in 99.78% of patients with successful restoration of myocardial perfusion. Most patients required stent lengths of 50 mm (39.52%) or 60 mm (38.21%). The TIMI flow grade improved to 3 after the procedure in 98.26% of patients (Figure 1). There were no cases of deployment failure.
Table 2. Lesion and procedural characteristics.
| Lesion characteristics | n=459 lesions/ 448 patients |
|---|---|
| Total no. of lesions treated with the study stent | 459 |
| Stenosis | |
| De novo | 455 (99.13) |
| In-stent | 4 (0.87) |
| ACC/AHA lesion classification | |
| Type A | 106 (23.09) |
| Type B1 | 87 (18.95) |
| Type B2 | 25 (5.45) |
| Type C | 241 (52.52) |
| Type of lesion | |
| Calcified | 11 (2.4) |
| CTO | 28 (6.1) |
| Diffuse | 51 (11.11) |
| Thrombus | 12 (2.61) |
| Other | 5 (1.09) |
| Lesion location | |
| LAD | 251 (54.68) |
| RCA | 164 (35.73) |
| LCx | 43 (9.37) |
| Other (LM to LAD) | 1 (0.22) |
| Procedural characteristics | n=459 lesions/ 448 patients |
| Stent length, mm | |
| 30 | 23 (5.02) |
| 40 | 79 (17.25) |
| 50 | 181 (39.52) |
| 60 | 175 (38.21) |
| Stent diameter, mm | |
| 2.75-2.25 | 138 (30.13) |
| 3.00-2.50 | 166 (36.24) |
| 3.50-3.00 | 150 (32.75) |
| 3.50-2.75 | 4 (0.87) |
| Procedural success | 447 (99.78) |
| Device success | 447 (99.78) |
| Predilatation | 441 (96.08) |
| Post-dilatation | 432 (94.12) |
| Procedure access site | |
| Femoral | 180 (40.18) |
| Radial | 267 (59.60) |
| Other (ulnar) | 1 (0.22) |
| Procedure access location | |
| Right | 445 (99.33) |
| Left | 3 (0.67) |
| CTO: chronic total occlusion; LAD: left anterior descending artery; LCx: left circumflex artery; LM: left main; RCA: right coronary artery | |
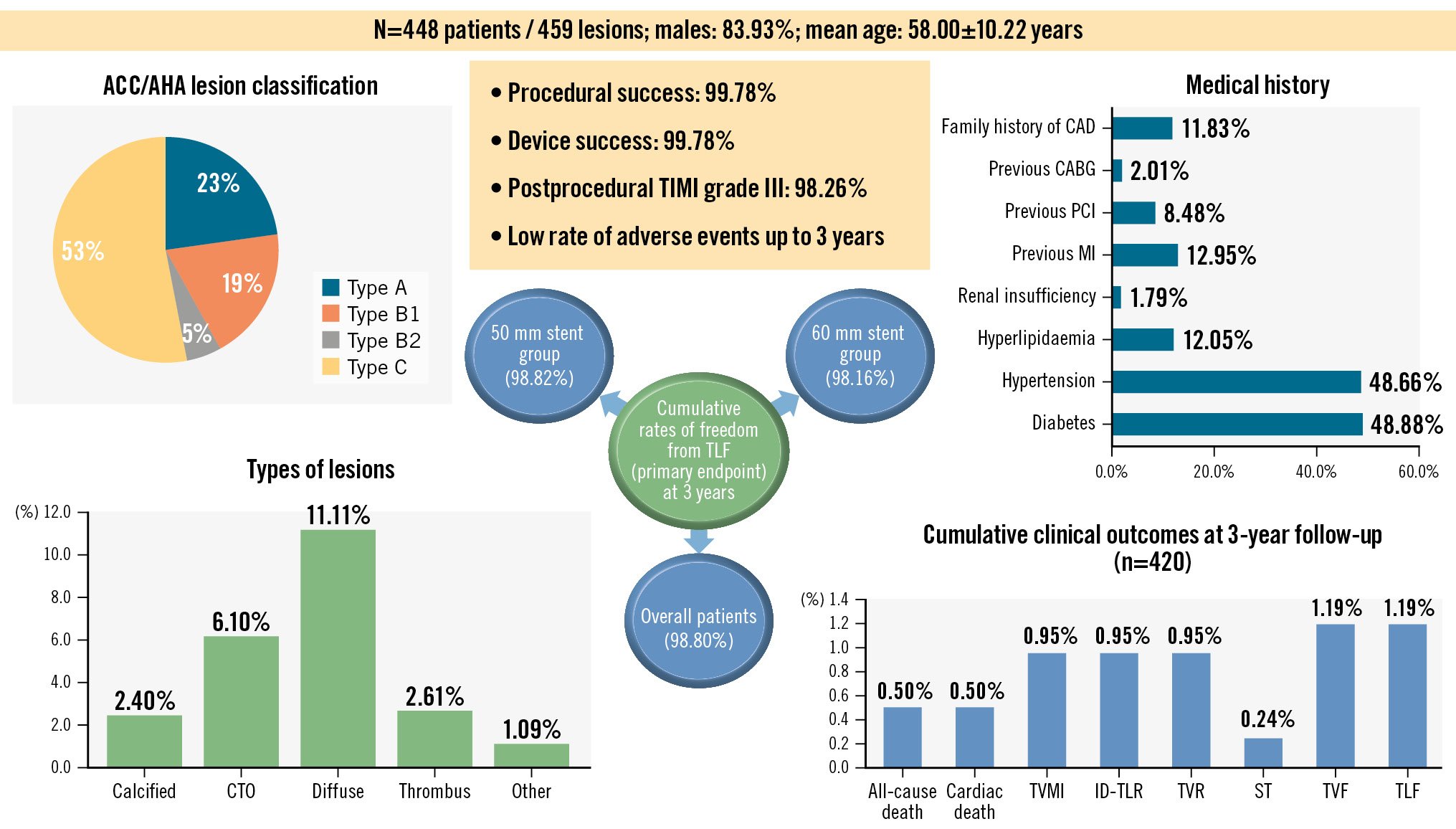
Central illustration. Morph India study: BioMime Morph for very long coronary lesions. CABG: coronary artery bypass graft; CAD: coronary artery disease; CTO: chronic total occlusion; ID-TLR: ischaemia-driven target lesion revascularisation; MI: myocardial infarction; PCI: percutaneous coronary intervention; ST: stent thrombosis; TIMI: Thrombolysis in Myocardial Infarction; TLF: target lesion failure; TVMI: target vessel-related myocardial infarction; TVF: target vessel failure; TVR: target vessel revascularisation
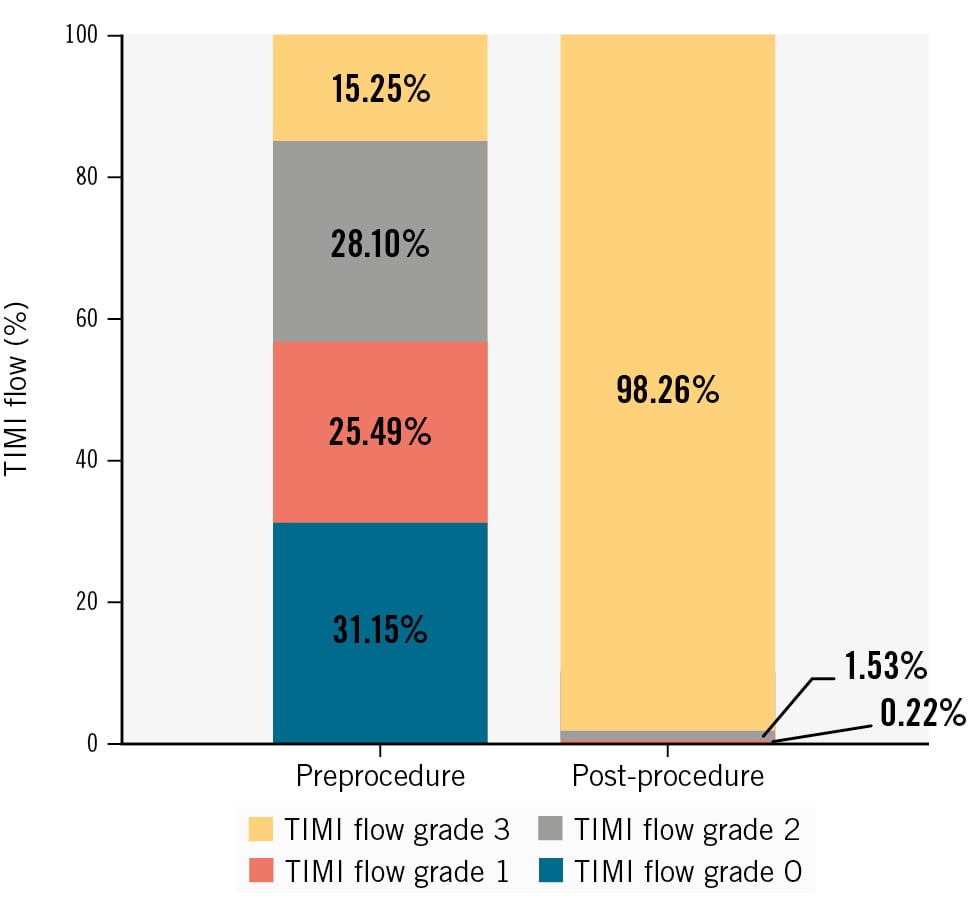
Figure 1. Changes in TIMI flow after the index procedure (n=448). Values are presented as %. TIMI: Thrombolysis in Myocardial Infarction
Early safety
The in-hospital and early (30-day) outcomes were satisfactory, with only one death in a patient who underwent primary PCI with the study device. The death was registered as a case of cardiac death prior to the 1-month follow-up (Table 3). The primary endpoint of freedom from TLF at 12 months was achieved in 99.31% of patients. One patient had TVMI with evidence of ST and required a TLR (Table 3). Another patient experienced chest pain following the index PCI and underwent repeat coronary artery angiography, which revealed an ISR lesion requiring TLR. The patient remained stable and completed the 1-year follow-up without experiencing any cardiovascular morbidity. Following completion of the 6-month follow-up, 3 cases each of TVMI, ID-TLR and TVR (including ID-TVR) were reported (0.68%). One incidence of definite ST (0.22%) occurred in a patient who underwent TLR.
Table 3. Clinical outcomes of the study population up to the 3-year follow-up.
| Events | In-hospital n=448 | 1 month n=446 | 6 months n=441 | 12 months n=434 | 24 months n=420 | 36 months n=420 |
|---|---|---|---|---|---|---|
| All-cause death | 0 (0) | 1 (0.22) | 1 (0.23) | 1 (0.23) | 1 (0.24) | 2 (0.5) |
| Cardiac death | 0 (0) | 1 (0.22) | 1 (0.23) | 1 (0.23) | 1 (0.24) | 2 (0.5) |
| Non-cardiac death | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| TVMI | 1 (0.22) | 1 (0.22) | 3 (0.68) | 3 (0.69) | 4 (0.95) | 4 (0.95) |
| ID-TLR | 1 (0.22) | 1 (0.22) | 3 (0.68) | 3 (0.69) | 4 (0.95) | 4 (0.95) |
| TVR (inclusive of ID-TVR) | 1 (0.22) | 1 (0.22) | 3 (0.68) | 3 (0.69) | 4 (0.95) | 4 (0.95) |
| ST | 1 (0.22) | 1 (0.22) | 1 (0.23) | 1 (0.23) | 1 (0.24) | 1 (0.24) |
| Definite | 1 (0.22) | 1 (0.22) | 1 (0.23) | 1 (0.23) | 1 (0.24) | 1 (0.24) |
| Probable | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Possible | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| TVF | 1 (0.22) | 1 (0.22) | 3 (0.68) | 3 (0.69) | 4 (0.95) | 5 (1.19) |
| TLF | 1 (0.22) | 1 (0.22) | 3 (0.68) | 3 (0.69) | 4 (0.95) | 5 (1.19) |
| Freedom from TLF | 447 (99.78) | 445 (99.78) | 438 (99.32) | 431 (99.31) | 416 (99.05) | 415 (98.80) |
| Values are presented as n (%). TVF is a composite of cardiac death, TVMI and ID-TVR; TLF is a composite of cardiac death, TVMI and ID-TLR. ID-TLR: ischaemia-driven target lesion revascularisation; ID-TVR: ischaemia-driven target vessel revascularisation; ST: stent thrombosis; TLF: target lesion failure; TVF: target vessel failure; TVMI: target vessel-related myocardial infarction; TVR: target vessel revascularisation | ||||||
Safety outcomes up to 3-year follow-up
The rates of freedom from TLF were 99.05% and 98.80% at the 2-year and 3-year timepoints, respectively. At the 3-year follow-up, there were 2 cardiac deaths. Four cases each of TVMI (0.95%), ID-TLR (0.95%), and TVR (0.95%; including ID-TVR) events occurred during the 3-year period. These 4 events included the in-hospital incidence of TVMI, ID-TLR, TVR, and ID-TVR in one patient, two patients at the 6-month follow-up, and one patient who experienced TVMI, ID-TLR, TVR and ID-TVR between the 1-year and 2-year follow-ups. Overall, the revascularisation rates were quite low for the 3-year follow-up period, including only 1.19% for TVF and TLF (n=5 for each) and a visibly low frequency of ST (0.24%, n=1) (Table 3, Central illustration).
Subgroup analysis of patients requiring 50 mm and 60 mm stents
In all, 355 patients had 356 extra-long lesions; 181 patients required 50 mm stents, and 174 required 60 mm stents. The baseline characteristics of these patients are shown in Table 4. Approximately 50% of patients had TIMI flow grade 1 or 2 before the procedure. The stents tapering from 3.00 mm proximally to 2.50 mm distally were used in 38.67% of patients in the 50 mm subgroup and in 34.86% of patients in the 60 mm subgroup. Post-procedure, TIMI flow grade 3 was achieved in 98.34% and 97.71% of patients in the respective subgroups (Table 4).
In patients who required 50 mm and 60 mm stents, there were no in-hospital or early deaths (Table 5). The rates of TVMI, ID-TLR, and TVF at the 6-month follow-up remained at 1.12% in the 50 mm subgroup. At the 3-year follow-up, the frequencies of all-cause death and cardiac death remained considerably low at 0.59% in the 50 mm subgroup (Table 5). Freedom from TLF at 1 year was achieved in 98.86% of patients; this was 98.82% at 3 years. In the 60 mm subgroup, 100% freedom from TLF was achieved at the 30-day follow-up; this was 98.16% at 3 years. There were no cases of ST (early or late) in the 60 mm subgroup, whereas 3 events of TVF (1.84%) and 2 events each of TVMI, TVR (including ID-TVR) and ID-TLR (all 1.23%) were observed (Table 5).
Table 4. Baseline cardiac status, lesion and procedural characteristics of patients with 50 mm and 60 mm stent lengths.
| Baseline characteristics | Stent length 50 mm (patients/lesions=181/181) | Stent length 60 mm (patients/lesions=174/175) |
|---|---|---|
| Patient characteristics | ||
| Age, years | 57.70±9.83 | 58.29±10.55 |
| Sex | ||
| Male | 157 (86.74) | 148 (85.06) |
| Female | 24 (13.26) | 26 (14.94) |
| Cardiac status | ||
| Stable angina | 38 (20.99) | 24 (13.79) |
| Unstable angina | 59 (32.6) | 58 (33.33) |
| STEMI | 61 (33.7) | 65 (37.36) |
| Non-STEMI | 22 (12.15) | 20 (11.49) |
| Silent ischaemia/asymptomatic | 1 (0.55) | 7 (4.02) |
| Lesion characteristics | ||
| Lesions per patient | 1.00 | 1.01 |
| Stenosis | ||
| De novo | 179 (98.90) | 173 (98.86) |
| In-stent | 2 (1.10) | 2 (1.14) |
| Lesion class | ||
| A | 48 (26.52) | 45 (25.71) |
| B1 | 34 (18.78) | 36 (20.57) |
| B2 | 11 (6.08) | 11 (6.29) |
| C | 88 (48.62) | 83 (47.43) |
| Lesion type | ||
| Calcified | 4 (2.21) | 6 (3.43) |
| CTO | 11 (6.08) | 8 (4.57) |
| Diffuse | 20 (11.05) | 21 (12.00) |
| Other | 3 (1.66) | 2 (1.14) |
| Thrombus | 5 (2.76) | 6 (3.43) |
| TIMI flow preprocedure | ||
| 0 | 54 (29.83) | 66 (37.71) |
| 1 | 35 (19.34) | 56 (32.00) |
| 2 | 57 (31.49) | 30 (17.14) |
| 3 | 35 (19.34) | 23 (13.14) |
| Lesion location | ||
| RCA | 63 (34.81) | 79 (45.14) |
| LAD | 99 (54.70) | 93 (53.14) |
| LCx | 18 (9.94) | 3 (1.71) |
| Other | 1 (0.55) | 0 (0) |
| Procedural characteristics | ||
| Total number of lesions treated with BioMime Morph | 181 | 175 |
| Total number of BioMime Morph stents used | 181 | 175 |
| TIMI flow post-procedure | ||
| 0 | 0 (0) | 0 (0) |
| 1 | 0 (0) | 1 (0.57) |
| 2 | 3 (1.66) | 3 (1.71) |
| 3 | 178 (98.34) | 171 (97.71) |
| Predilatation | 173 (95.58) | 168 (96.00) |
| Post-dilatation | 173 (95.58) | 160 (91.43) |
| Stent diameter, mm | ||
| 2.75-2.25 | 53 (29.28) | 54 (30.86) |
| 3.00-2.50 | 70 (38.67) | 61 (34.86) |
| 3.50-3.00 | 55 (30.39) | 60 (34.29) |
| 3.50-2.75 | 3 (1.66) | 0 (0) |
| Procedure access site | ||
| Femoral | 74 (40.88) | 56 (32.18) |
| Radial | 107 (59.12) | 117 (67.24) |
| Other | 0 (0) | 1 (0.57) |
| Ulnar | 0 (0) | 1 (0.57) |
| Procedure access location | ||
| Right | 178 (98.34) | 174 (100) |
| Left | 3 (1.66) | 0 (0) |
| Values are presented as mean±SD or n (%). CTO: chronic total occlusion; LAD: left anterior descending artery; LCx: left circumflex artery; RCA: right coronary artery; SD: standard deviation; STEMI: ST-segment elevation myocardial infarction; TIMI: Thrombolysis in Myocardial Infarction | ||
Table 5. Three-year clinical outcomes of patients with extra-long stents (50 mm and 60 mm).
| 50 mm length stent group | ||||||
|---|---|---|---|---|---|---|
| Events | In-hospital n=181 | 1 month n=181 | 6 months n=179 | 12 months n=175 | 24 months n=169 | 36 months n=169 |
| All-cause death | 0 (0) | 1 (0.55) | 1 (0.56) | 1 (0.57) | 1 (0.59) | 1 (0.59) |
| Cardiac death | 0 (0) | 1 (0.55) | 1 (0.56) | 1 (0.57) | 1 (0.59) | 1 (0.59) |
| Non-cardiac death | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| TVMI | 1 (0.55) | 1 (0.55) | 2 (1.12) | 2 (1.14) | 2 (1.18) | 2 (1.18) |
| ID-TLR | 1 (0.55) | 1 (0.55) | 2 (1.12) | 2 (1.14) | 2 (1.18) | 2 (1.18) |
| TVR (inclusive of ID-TVR) | 1 (0.55) | 1 (0.55) | 2 (1.12) | 2 (1.14) | 2 (1.18) | 2 (1.18) |
| ST | 1 (0.55) | 1 (0.55) | 1 (0.56) | 1 (0.57) | 1 (0.59) | 1 (0.59) |
| Definite | 1 (0.55) | 1 (0.55) | 1 (0.56) | 1 (0.57) | 1 (0.59) | 1 (0.59) |
| Probable | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Possible | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| TVF | 1 (0.55) | 1 (0.55) | 2 (1.12) | 2 (1.14) | 2 (1.18) | 2 (1.18) |
| TLF | 1 (0.55) | 1 (0.55) | 2 (1.12) | 2 (1.14) | 2 (1.18) | 2 (1.18) |
| Freedom from TLF | 180 (99.45) | 180 (99.45) | 177 (98.88) | 173 (98.86) | 167 (98.82) | 167 (98.82) |
| 60 mm length stent group | ||||||
| n=174 | n=173 | n=172 | n=169 | n=163 | n=163 | |
| All-cause death | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.61) |
| Cardiac death | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.61) |
| Non-cardiac death | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| TVMI | 0 (0) | 0 (0) | 1 (0.58) | 1 (0.59) | 2 (1.23) | 2 (1.23) |
| ID-TLR | 0 (0) | 0 (0) | 1 (0.58) | 1 (0.59) | 2 (1.23) | 2 (1.23) |
| TVR (inclusive of ID-TVR) | 0 (0) | 0 (0) | 1 (0.58) | 1 (0.59) | 2 (1.23) | 2 (1.23) |
| ST | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Definite | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Probable | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Possible | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| TVF | 0 (0) | 0 (0) | 1 (0.58) | 1 (0.59) | 2 (1.23) | 3 (1.84) |
| TLF | 0 (0) | 0 (0) | 1 (0.58) | 1 (0.59) | 2 (1.23) | 3 (1.84) |
| Freedom from TLF | 174 (100) | 173 (100) | 171 (99.42) | 168 (99.41) | 161 (98.77) | 160 (98.16) |
| Values are presented as n (%). ID-TLR: ischaemia-driven target lesion revascularisation; MI: myocardial infarction; ST: stent thrombosis; TLF: target lesion failure; TVF: target vessel failure; TVMI: target vessel-related myocardial infarction; TVR: target vessel revascularisation | ||||||
Analysis of quantitative coronary angiographic data
An angiographic follow-up was conducted among a representative group of patients to ascertain the stent apposition and expected increase in luminal diameters. The QCA data from the mean 9.2-month follow-up were assessed to evaluate the angiographic LLL, which is a robust surrogate marker of the risk for ISR and TLR. In this study, a moderate level of LLL was observed, both in the stented segment (0.35±0.11 mm) and in the diseased coronary segment (0.29±0.23 mm). In addition, significant improvements in the in-device MLD were observed at the postprocedural and the 9-month angiographic follow-up (baseline vs follow-up: 0.63±0.42 mm vs 2.13±0.37 mm; p<0.001) (Table 6, Supplementary Figure 3).
Table 6. Quantitative coronary angiography at the mean 9.2-month follow-up.
| Parameters | Preprocedure | Post-procedure | 9.2-month follow-up | p-value |
|---|---|---|---|---|
| In-device | ||||
| MLD, mm | 0.63±0.42 | 2.48±0.38 | 2.13±0.37 | <0.001 |
| LLL, mm | – | – | 0.35±0.11 | – |
| In-segment | ||||
| MLD, mm | 0.59±0.42 | 2.26±0.42 | 1.97±0.38 | <0.001 |
| LLL, mm | – | – | 0.29±0.23 | – |
| Values are mean±SD. LLL: late lumen loss; MLD: minimal luminal diameter; SD: standard deviation | ||||
Discussion
Our study shows that the BioMime Morph SES is safe and effective in treating very long (length >26 mm to ≤56 mm) coronary lesions in native coronary arteries with a reference vessel diameter of 2.25 mm to 3.50 mm up to 3 years. There were low rates of all-cause mortality (0.5%) and stent thrombosis (0.24%). Overall, 98.80% of patients had freedom from TLF at 3 years. Similar outcomes were seen even in the subset of patients who required very long stents of 50 mm and 60 mm in length. This is despite the fact that more than 50% of the cohort had TIMI grade 0 and 1 before the procedure, and almost 80% of our cohort had very long lesions requiring stent lengths of 50 mm and 60 mm. Previously, the Interventional Cardiology Research In-Cooperation Society-Drug-Eluting Stents (IRIS-DES) registry showed that the stent length of 43.0 mm was the differential cutoff for predicting the risk of TVF with second-generation DES21. However, our outcomes were much superior to these.
Previous studies have reported outcomes with the BioMime Morph SES in long coronary lesions. Among the initial studies was one by Matchin et al, who reported a 98.8% clinical success rate. Restenosis was seen in 10.4% of patients at 12 months16. Zivelonghi et al reported a procedural success rate of 89% and a cardiac death rate of 2% at 1 year, with a TVR rate of 4.1%22. Subsequently, Patted et al reported a cumulative major adverse cardiac events (MACE) incidence of 2.0% and ST of 0.3% at 12-month follow-up with the same stent9. Podolec et al reported a MACE rate of 0% at 3, 6, and 12 months in a small cohort of 32 patients15. Jurado-Román et al reported a MACE rate of 6.2% and ST rate of 0% at 20 months10, while Sharma et al reported a 1-year MACE rate of 4.7%12. Miñana et al used the BioMime Morph SES for the treatment of chronic total occlusion (CTO) and reported a procedural success rate of 94.4%. During a median follow-up of 330 (interquartile range 149-551) days, repeat PCI in the target vessel was performed in 3.7% of patients [13. Our outcomes are similar or superior to those of these previous studies. However, all previous studies reported only immediate post-procedure or 1-year outcomes, except one that reported 20-month outcomes. Ours is the first study to report 3-year outcomes with the BioMime Morph SES in real-world settings.
Kang et al previously compared the 12-month relative performance of six different types of DES for de novo long (≥25 mm) coronary artery lesions from 3 randomised clinical trials23. The stents compared were XIENCE V (Abbott), PROMUS and PROMUS Element (both Boston Scientific), Endeavor Resolute (Medtronic), Nobori (Terumo), and CYPHER (Cordis Corporation). The comparative outcomes of these stents with that of our study are shown in Supplementary Table 1 and indicate that our outcomes were superior to all 6 stents.
In the randomised, multicentre IVUS-XPL trial including long lesions, Hong et al reported lower rates of early adverse events (≤ 1 year) in the intravascular ultrasound (IVUS)-guided PCI group than in the angiography-guided PCI group with the XIENCE Prime everolimus-eluting stent (EES; Abbott) including cardiac death (0.4% and 0.7%; p=0.480), ID-TLR (2.5% and 5.0%; p=0.020), definite or probable ST (0.3% and 0.3%; p=1.000), and MACE (2.9% and 5.8%; p=0.007)2425. In a prospective, multicentre, observational study of the Resolute Integrity zotarolimus-eluting stent (ZES; Medtronic), Park et al reported 0.5% cardiac death, 0.3% ST, 1.7% clinically driven (CD)-TLR, and 1.9% CD-TVR at 1 year26. The outcomes of the overall population, as well as for the 50 mm and 60 mm groups, in the current study are comparable to or numerically better than the outcomes reported in these studies, despite the lack of IVUS imaging analysis and the inclusion of patients with CTO and ISR lesions, who were not included in the IVUS-XPL trial. The LONG-DES-IV randomised trial compared the efficacy and safety of the Resolute ZES and SES in patients with de novo long coronary lesions. The device success rate with both devices (99.8%) was comparable with the current study (99.78%). The SES group in that study showed higher incidences of all-cause death (1.2%), cardiac death (0.8%), non-cardiac death (0.4%), definite or probable ST (0.8%), TLR (0.8%), TVR (0.8%), MI (13.2%), and TLF (16.0%) than the 50 mm and 60 mm groups of our study at the 1-month and 1-year follow-ups27. It is important to note that the LONG-DES-IV trial utilised SES with lengths up to only 33 mm, which likely required the use of multiple stents for single long lesions. In contrast, our study utilised tapered SES of very long lengths (50 mm and 60 mm), eliminating the need for multiple stents per lesion. This difference in stent length usage may have contributed to the lower incidence of adverse outcomes observed in our study.
Paszek et al reported a 13.4% incidence of a device-oriented composite endpoint (DOCE), which included cardiac death, TVMI, or target lesion restenosis, at a median follow-up of 831 days (range: 390-1,373 days). This rate was higher than the 3-year TLF rate in our study (1.19%) with a similar definition. The probable reason for this difference may be the higher percentage of patients with chronic kidney insufficiency (15.2%) in the Paszek et al study compared to our study (renal insufficiency: 1.79%), as this was the strongest predictor of the DOCE28. Additionally, the use of overlapping stents was more common in their study (83.1%) compared to our study (0.22%)28. Furthermore, the BioMime Morph device has shown favourable outcomes without any periprocedural complications in bifurcation lesions515.
PCI in the unprotected left main artery (ULM) is technically challenging because of the diameter differences (tapering) in the ULM and its side branches. These calibre discrepancies may lead to the risk of restenosis and ST due to stent undersizing in the ULM and dissection or perforation due to oversizing in the side branches29. The tapered design of stents like BioMime Morph SES and XIENCE Skypoint EES (Abbott) has been devised to address the challenges of treating such types of tapered vessels by offering improved adaptability to the vessel wall. The BioMime Morph SES has shown favourable outcomes in tapered left main bifurcation lesions at a median follow-up of 17 months5. Additionally, Xposition SES (STENTYS) has also been utilised in treating tapered lesions, especially in left main bifurcation lesions3031. It should be noted that the BioMime Morph SES has shown better outcomes in left main lesions as compared to Xposition SES. However, the patient population was lower in the study of BioMime Morph SES, and hence, studies with larger patient populations and longer follow-ups are warranted.
Limitations
The primary limitation of the study is that only a moderate sample size was included. Nonetheless, the sample size of the study is adequate to estimate the clinical outcomes of PCI with the novel device, considering that a moderately complex study population was enrolled including significantly comorbid patients with strong cardiovascular risk factors such as diabetes, hypertension, history of MI and family history of CAD. Even patients who had undergone coronary artery bypass graft or PCI previously were included. The second limitation is the lack of intravascular imaging assessments of lesions; however, QCA analysis was performed at a mean follow-up of 9.2 months. It has been argued in contemporary literature that long-term angiographic follow-up should be included in clinical studies focused on PCI outcomes. However, in the current era of second-generation DES when significantly low incidences of late ST are observed in routine clinical practice, angiographic evaluation of patients implanted with stents is not mandated for long-term post-PCI assessments. Therefore, clinically indicated angiographic follow-ups are recommended. Additionally, this study did not utilise physiological assessment methods to determine the functional impact of stenosis, which could have influenced the outcomes. Another limitation of this post-marketing surveillance, observational study is the possibility of selection bias, as patients were not randomly selected or compared to a control group. The other limitation was that the association between different dual antiplatelet therapy regimens and the clinical prognosis of PCI with the study device could not be established, which was partly because of the lack of heterogeneity in the outcomes data. Nevertheless, the study outcomes are well generalisable for the Asian populations in whom PCI is clinically indicated.
Conclusions
The promising long-term results of BioMime Morph provide acceptable clinical evidence for a wide variety of CAD lesions in which vessels showing significant vessel taper were treated using 30 mm, 40 mm, 50 mm or 60 mm stents. Currently, our data support the use of the BioMime Morph sirolimus-eluting coronary stent system in an all-comers CAD population. Larger comparative studies with other stent platforms (cobalt-chromium, platinum-chromium, and nickel-titanium) are anticipated to establish the clinical performance of this unique, long, tapered stent system.
Impact on daily practice
BioMime Morph has a unique hybrid stent design, combining closed cells at the two distal ends and open cells in the centre. This stent is designed to sufficiently conform to the vasculature and reduce the chances of edge dissections and vascular injury. Since BioMime Morph SES tapers in accordance with vascular architecture and covers vessel lengths up to 60 mm, it reduces the need for implanting >1 stent, thus lowering the risks of fracture and restenosis. The single-stenting strategy reduces procedural time and avoids unwarranted exposure to radiation and contrast media infusion, thus improving the procedural safety and increasing the cost-effectiveness of PCI. In the future, larger multinational studies with diverse patient populations need to be conducted to establish the efficacy and safety of this device.
Acknowledgements
The authors would like to thank the trial monitoring teams at the respective clinical sites and the members of the Clinical Research Department at Meril Life Sciences Pvt. Ltd., whose constant efforts were essential in the completion of the study and contributed significantly to the publication of this manuscript.
Funding
The Morph India study was sponsored by Meril Life Sciences Pvt. Ltd., India.
Conflict of interest statement
D. Davidson has received grants and research support from Meril Life Sciences, Abbott, and Medtronic India. K. Parikh has served as an investigator for United Therapeutics. A. Thakkar is a full-time employee of Meril Life Sciences Pvt. Ltd., India. The other authors have no known conflicts of interest to declare.

