Introduction
Vascular access techniques in coronary angiography and percutaneous coronary intervention (PCI) have progressed rapidly over the last two decades. Guidelines recommend the transradial artery (TRA) as a first-line access site due to lower rates of bleeding and vascular complications and reduced mortality in acute coronary syndromes compared to the femoral artery12. One limitation of TRA access is radial artery occlusion (RAO), which may preclude future arterial access of the ipsilateral limb34.
The distal transradial artery (dTRA) approach has gained traction amongst some cardiologists since it was propelled into the mainstream following a report in 2017 by Ferdinand Kiemeneij5. Ergonomic advantages are notable especially when accessing the left radial or in obese patients, as well as in those with limited supination at the wrist. Benefits also include reduced vascular complications, including proximal RAO and haematoma formation6. Moreover, in the event of distal RAO, the ipsilateral radial artery may still be accessed proximally78.
Puncturing the smaller distal radial artery is more challenging, however, and requires more time, experience, and skill910. Hypothetically, the maximum diameter size of the catheter that may be used for PCI is also reduced, as the dTRA diameter is approximately 80% of that of the TRA11. Subsequently, the feasibility of dTRA access requires closer examination, particularly in the context of large-bore coronary artery PCI. Whilst advancing age, female sex, and short stature have been cited as predictors of transradial approach failure1213, it is unclear whether these are also implicated in patients for whom crossover is required following failed dTRA catheterisation. Little is known about the predictors of dTRA puncture failure, and this may help develop a selective approach for dTRA access. We evaluated the feasibility and safety of the dTRA as a first-line access site in consecutive patients from metropolitan hospitals in New South Wales, Australia.
Methods
Consecutive patients from three New South Wales hospitals who underwent coronary angiography and PCI via the dTRA, between November 2019 and December 2023, were included in the present study. Participant data included age, sex, smoking status, presence of hypertension, hyperlipidaemia or diabetes, use of anticoagulants or antiplatelets, weight, height, body mass index (BMI), creatinine level, and estimated glomerular filtration rate (eGFR). The primary outcome was first pass success, defined as successful radial artery cannulation with a single skin puncture; this definition excludes multiple attempts at needle repositioning but includes minor needle adjustments and multiple attempts at wire passage within the initial puncture site (typically guided by ultrasound). Overall success was defined as successful completion of coronary angiogram or PCI using the dTRA puncture, without necessitating crossover. Safety data included major bleeding and vascular complications as per Bleeding Academic Research Consortium (BARC)-2/Valve Academic Research Consortium (VARC)-2 criteria, access crossover, radial artery vasospasm, arterial patency at follow-up, major adverse cardiovascular events (MACE: myocardial infarction, stroke, or death within 30 days), and need for hospital readmission at 30 days and 6 months. Vasospasm was defined as an abrupt and temporary narrowing of the artery, associated with an inability to manipulate the guidewire or catheter in a smooth and painless manner, including difficulty in removing the sheath in a similar way at the end of the procedure14.
Ethical considerations
A multicentre cohort study was performed and registered within the Central Coast Local Health District (CCLHD) Human Research Ethics Committee (HREC; registration number 0722-057C). A low-to-negligible risk quality-improvement initiative approval was provided by the HREC.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS) version 28.0.1.1 (IBM) was used to analyse data. Frequencies, means, and standard deviations were used to describe the patient demographics. Normality was assessed using the Kolmogorov-Smirnov test. Univariate associations were analysed using the χ² statistical test. Variables that were deemed clinically relevant or hypothesised to be associated with success at first puncture were used in the multivariate logistic regression analysis. The resulting odds ratio (OR) values and the significance of each variable in relation to technical success were obtained. Additionally, percentages of correctly classified cases were calculated, the Omnibus coefficient test was used to assess the acceptance of the model, and the Hosmer-Lemeshow test was utilised to verify adjustment to the model; these analyses were conducted for the multivariate logistic regression model. The enter selection process was employed to include variables in the model. Statistical test decisions were based on a significance level of 5% (p<0.05), and 95% confidence intervals were used.
Results
A total of 1,692 patients were included in the study. The mean age was 70.6±10.5 years, and 59% were male. The mean weight, height and BMI were 86.3±25.4 kg, 169.4±14.4 cm, and 31.0±7.0 kg/m2, respectively (Table 1). Main comorbidities included diabetes (23.1%), hyperlipidaemia (83.5%), hypertension (77.4%), and active or former smoking (36.8%). Anticoagulants and antiplatelets used in the 24 hours immediately prior to the procedure included warfarin (0.2%), direct oral anticoagulants (DOACs [9.5%]), single antiplatelet with either aspirin, clopidogrel, or ticagrelor (33.5%) and dual antiplatelets (38.2%). The mean creatinine and eGFR were 89.3 μmol/L and 70.7 mL/min/1.73 m2, respectively.
Procedural outcomes can be seen in Table 2. The right dTRA was accessed in 85.4% (n=1,445), and ultrasound guidance was performed in 99.7% of cases (n=1,687). The median sheath size was 6 (interquartile range 5-6) Fr with a range of 4-7.5 Fr. Vasospasm occurred in 52 cases (3.1%). A total of 64.8% of cases were diagnostic (n=1,097), and PCI occurred in 35.3% of cases (n=595). Imaging guidance was used for 16.9% of PCI procedures (n=285), predominantly intravascular ultrasound (IVUS; n=275 [96%]) and optical coherence tomography (OCT; n=10 [4%]).
Table 1. Demographic data.
| Demographic | n=1,692 |
|---|---|
| Age, years | 70.6±10.5 |
| Weight, kg | 86.3±25.4 |
| Height, cm | 169.4±14.4 |
| BMI, kg/m2 | 31.0±7.0 |
| Creatinine, µmol/L | 89.3±44.9 |
| eGFR, mL/min/1.73 m2 | 70.7±17.7 |
| Male | 993 (58.7) |
| Current smoker | 140 (8.3) |
| Former smoker | 483 (28.5) |
| Hyperlipidaemia | 1,413 (83.5) |
| Hypertension | 1,310 (77.4) |
| Diabetes | 391 (23.1) |
| Warfarin use within 24 hoursprior to procedure | 4 (0.2) |
| DOAC or heparin use within 24 hours prior to procedure | 160 (9.5) |
| Antiplatelet use within 24 hours prior to procedure | Monotherapy: 567 (33.5)Dual therapy: 647 (38.2) |
| Continuous variables are represented as mean±standard deviation. Categorical variables are represented as n (%). BMI: body mass index; DOAC: direct oral anticoagulant; eGFR: estimated glomerular filtration rate | |
Table 2. Procedural outcomes.
| Procedural outcomes | n=1,692 |
|---|---|
| Success on first puncture | 1,560 (92.2) |
| Number of punctures | 1.1±0.3 |
| Crossover required | 107 (6.3) |
| Contralateral dTRA | 22 (20.6) |
| Ipsilateral TRA | 78 (72.9) |
| Femoral | 6 (5.6) |
| Ulnar | 1 (0.9) |
| Right-sided puncture | 1,445 (85.4) |
| Ultrasound-guided puncture | 1,687 (99.7) |
| Vasospasm | 52 (3.1) |
| Indication | |
| Diagnostic | 1,097 (64.8) |
| PCI | 595 (35.2) |
| Intracoronary imaging | |
| IVUS | 275 (16.3) |
| OCT | 10 (0.6) |
| Operator experience | |
| Attending interventional cardiologist (≥4 years’ experience) |
1,402 (82.9) |
| Cardiology fellow/registrar (≤3 years’ experience) |
290 (17.1) |
| Radiation dose, mGy | 38.7±28.8 |
| Contrast, mL | 92.8±54.5 |
| GTN, mg | 351.1±263.1 |
| Sheath size, Fr | 6 (5-6) |
| Midazolam, mg | 1 (1-2) |
| Fentanyl, mcg | 50 (50-75) |
| Categorical variables are represented as n (%). Normally distributed continuous variables are represented as mean±standard deviation. Non-normally distributed data are represented as median (IQR). dTRA: distal transradial artery; GTN: glyceryl trinitrate; IQR: interquartile range; IVUS: intravascular ultrasound; OCT: optical coherence tomography; PCI: percutaneous coronary intervention; TRA: transradial artery | |
Primary outcome
First pass technical success was 92.2% (n=1,560). Successful puncture of the ipsilateral dTRA on the second puncture was observed in 1.5% (n=25), and crossover was required in 6.3% (n=107) (contralateral dTRA [n=22], proximal transradial [n=78], femoral [n=6], ulnar [n=1]) (Central illustration). Thus, the overall success rate was 93.7% (n=1,585). The mean number of punctures was 1.1±0.3 (Table 2). Reasons for failed puncture were categorised into access-related (n=54) and clinical/patient-related (n=14) factors (Table 3).
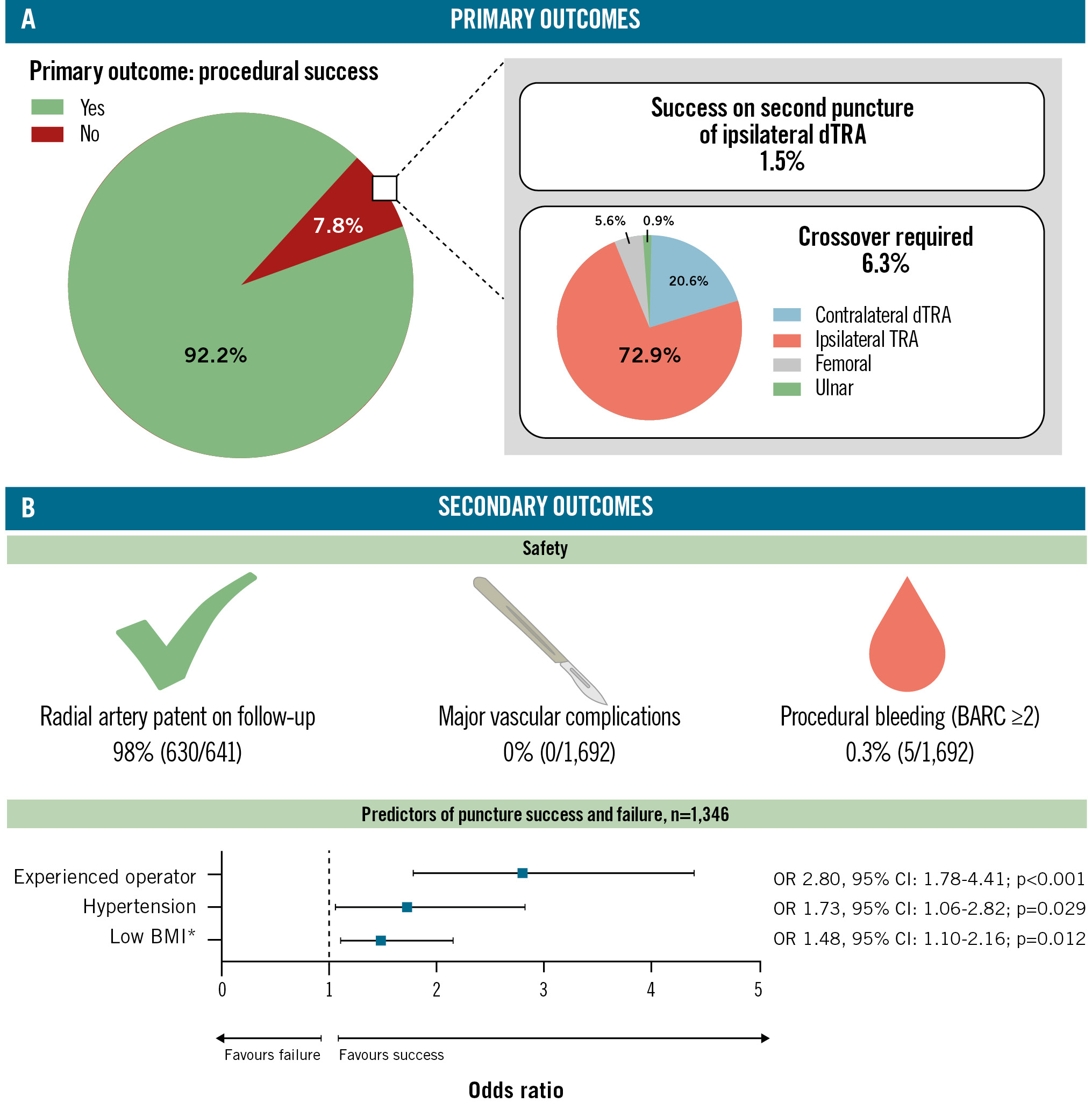
Central illustration. Distal radial first: primary and secondary outcomes. A) Primary outcomes; (B) secondary outcomes. *The OR for BMI has been exponentiated to the power of 10 to better demonstrate clinical effect. BARC: Bleeding Academic Research Consortium; BMI: body mass index; CI: confidence interval; dTRA: distal transradial artery; OR: odds ratio; TRA: transradial artery
Table 3. Reasons for failed dTRA approach.
| Failed approach | n=132 |
|---|---|
| Access site-related factors | |
| Subintimal wire cannulation | 18 (13.6) |
| dTRA <1.5 mm diameter | 17 (12.9) |
| Radial artery spasm | 11 (8.3) |
| Atretic/occluded radial artery | 6 (4.5) |
| Retroflexed wire | 1 (0.8) |
| Overlying haematoma | 1 (0.8) |
| Clinical factors | |
| Subclavian tortuosity | 4 (3.0) |
| Sheath upsized for large-bore PCI | 3 (2.2) |
| Radial tortuosity | 2 (1.5) |
| Radial loop | 2 (1.5) |
| ARSA | 1 (0.8) |
| SBP >200 mmHg on sheath insertion | 1 (0.8) |
| Long catheter required for coronary angiogram/PCI due to tall stature | 1 (0.8) |
| Not specified | 64 (48.5) |
| Data are presented as n (%). ARSA: aberrant right subclavian artery; dTRA: distal transradial artery; PCI: percutaneous coronary intervention; SBP: systolic blood pressure | |
Secondary outcomes
Procedural bleeding requiring clinical treatment (BARC Type ≥2 within 24 hours) occurred in 0.3% (n=5). There were no vascular access site complications that required surgery or vascular interventions and no instances of hand/digital ischaemia (vascular access site and access-related complications: VARC-2 major: 0%; VARC-2 minor: 0%). Outpatient follow-up data for assessment of radial artery occlusion were available in 641 patients; the radial artery was patent in 98% (n=630) (Central illustration). Proximal occlusion and distal occlusion occurred in 0.1% and 0.4%, respectively. Thirty-day MACE occurred in 1.4% (n=24). Hospital readmission at 1 and 6 months occurred in 251 (14.8%) and 194 (11.5%) patients, respectively. Reasons prompting readmission were categorised into complications related to the initial procedure, deterioration of a known cardiac pathology, an elective cardiac procedure, and treatment of a non-cardiac comorbidity (Table 4). At 1 month, most readmissions were for elective procedures (n=146), followed by complications following the initial procedure and non-cardiac causes (each n=49), and, lastly, the deterioration of a known cardiac pathology (n=7). Most postprocedural complications were attributed to post-PCI chest pain/angina, dyspnoea, or arrhythmia. At 6 months, non-cardiac pathology was the predominant cause for readmission (n=84), followed by deterioration or recurrence of a known cardiac pathology (n=54) of which non-ST-segment elevation myocardial infarction and decompensated heart failure predominated, and then, elective cardiology procedures (n=51). Complications following the original procedure were low (n=5).
Table 4. Safety outcomes.
| Safety outcomes | n=1,692 |
|---|---|
| Major vascular complications (including digital/hand ischaemia) |
0 (0) |
| Procedural bleeding (BARC ≥2 within 24 hours) |
6 (0.4) |
| Haematoma formation | 29 (1.7) |
| Radial artery occlusion | 6 (0.4) |
| Proximal | 2 (0.1) |
| Distal | 6 (0.4) |
| 30-day MACE | 24/1,662 (1.4) |
| Hospital readmission due to access site bleeding |
0 (0) |
| Hospital readmission within 1 month | 251 (14.8) |
| Complication of initial procedure | 49 |
| Deterioration of known cardiac pathology | 7 |
| Elective cardiac procedure | 146 |
| Non-cardiac comorbidities | 49 |
| Hospital readmission within 6 months | 194 (11.5) |
| Complication of initial procedure | 5 |
| Deterioration of known cardiac pathology | 54 |
| Elective cardiac procedure | 51 |
| Non-cardiac comorbidities | 84 |
| Radial artery patent at follow-up | 630/641 (98.3) |
| Continuous variables are represented as mean±standard deviation. Categorical variables are represented as n, (%) or n/N (%). BARC: Bleeding Academic Research Consortium; MACE: major adverse cardiovascular events | |
Predictors of failure
A total of 1,346 valid cases were included in the analysis. No cases were left unselected. The logistic regression model predicted success at the first puncture with 92.1% accuracy, correctly classifying 1,239 cases as “yes” and 0 cases as “no”. The constant term in the model was significant (B=2.449; p<0.001; Exp(B)=11.579), indicating its influence on the outcome. The Omnibus tests of model coefficients yielded a χ² statistic of 21.330 with 11 degrees of freedom, indicating a significant overall model prediction (p=0.030). This suggests that the combination of predictor variables included in the model collectively contributes to the variance in the outcome variable. The Hosmer-Lemeshow test of goodness of fit yielded a non-significant result (χ²=4.119, df=8; p=0.846), suggesting a good fit of the model to the data.
Logistic regression demonstrated that hypertension (OR 1.74; p=0.026) and attending/consultant level operator (≥4 years’ experience; OR 2.80; p<0.001) were statistically significant independent predictors of procedural success, compared to normotensive patients and cardiology fellow/registrar level operator, respectively. A low BMI (OR 1.48; p=0.012) was also a statistically significant predictor of puncture success, reflecting a 4% increased chance of success per 1 unit decrease in BMI (kg/m2) or a 48% increased chance of success per 10 unit decrease in BMI (Central illustration, Table 5, Figure 1, Figure 2).
Table 5. Odds ratios, 95% confidence intervals and p-values of factors affecting puncture success, derived from the logistic regression model.
| Logistic regression | n=1,346 | ||
|---|---|---|---|
| Variable | p-value | Odds ratio | 95% CI |
| Female sex | 0.425 | 1.190 | 0.776-1.827 |
| Age | 0.342 | 0.990 | 0.971-1.010 |
| BMI | 0.012 | 1.480 | 1.102 – 2.161 |
| Diabetes | 0.462 | 0.840 | 0.527-1.338 |
| Hyperlipidaemia | 0.305 | 0.740 | 0.416-1.315 |
| Hypertension | 0.029 | 1.729 | 1.059-2.822 |
| GTN use | 0.591 | 0.857 | 0.487-1.506 |
| Current smoker* | 0.811 | 0.917 | 0.450-1.869 |
| Ex-smoker* | 0.823 | 0.949 | 0.599-1.504 |
| PCI performed† | 0.965 | 1.019 | 0.439-2.363 |
| Experienced operator | <0.001 | 2.803 | 1.782-4.407 |
| *Comparison group was “non-smokers”. †Comparison group was “PCI not performed” (diagnostic angiogram only). BMI: body mass index; CI: confidence interval; GTN: glyceryl trinitrate; PCI: percutaneous coronary intervention | |||
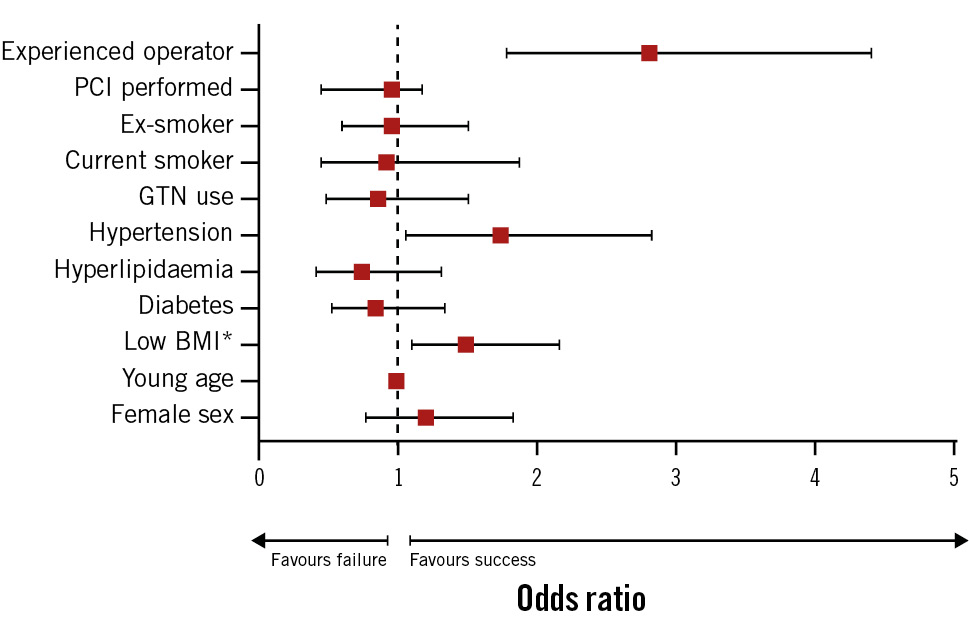
Figure 1. Odds ratios and 95% confidence intervals of factors affecting distal radial puncture success and failure. *The odds ratio for BMI has been exponentiated to the power of 10 to better demonstrate clinical effect. BMI: body mass index; GTN: glyceryl trinitrate; PCI: percutaneous coronary intervention
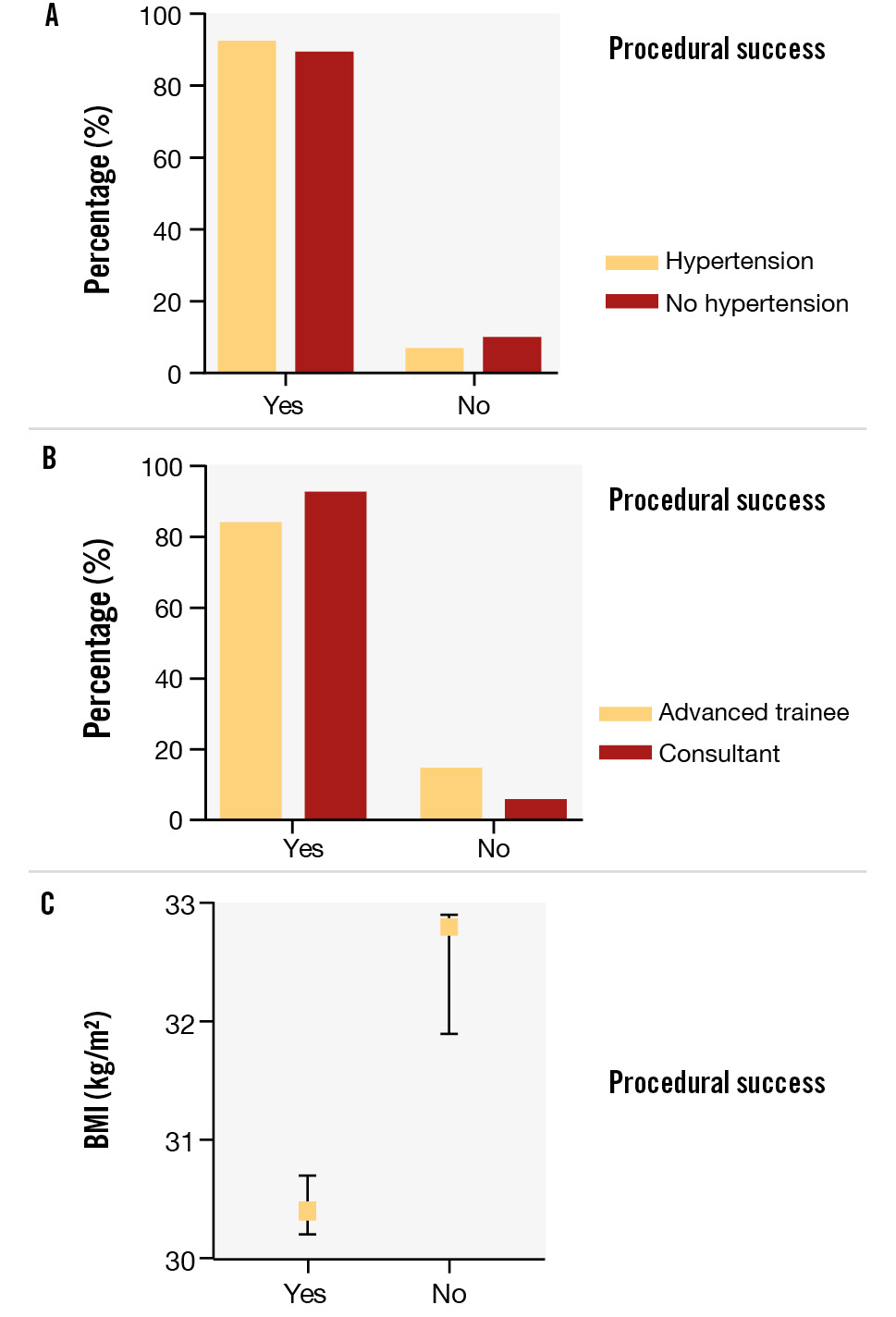
Figure 2. Procedural success demonstrated as a percentage: significant predictors per the logistic regression model. Procedural success expressed as a percentage in those with and without hypertension (A); in cardiology fellow/registrar versus attending/consultant operators (B); and as a function of mean body mass index (BMI) in kg/m2 (C). Error bars represent the standard error of the mean.
Discussion
This Australian multicentre study of 1,692 consecutive patients undergoing distal radial access confirms that distal transradial artery access is feasible and safe in an all-comer population undergoing coronary angiography and PCI. We noted high rates of technical success and no major vascular complications or instances of hand or digital ischaemia. Furthermore, this study provides valuable insights into the characteristics of the small subset of patients for whom dTRA puncture may be more challenging and might potentially be avoided.
Feasibility
The primary outcome of technical success, as defined by successful dTRA cannulation and sheath insertion with subsequent completion of either a coronary angiogram or PCI on the first attempt, was observed in 92.2% of cases. This rate was comparable to1516 or higher than17181920 the dTRA puncture success rates published in recent randomised controlled trials (RCTs) and observational studies. While the recently published KODRA trial16 reports a dTRA puncture success rate of 94.4%, this percentage includes initially successful dTRA punctures which later required crossover due to vessel tortuosity, vasospasm, vessel occlusion and anatomical patient factors. Following adjustment per our definition, the KODRA success rate appears closer to 92%, which is comparable to the present study. Additionally, crossover rates were reduced in comparison to the pooled event rate calculated in a recent meta-analysis21. Considering the reasons for technical failure documented, one-quarter would likely have been encountered in conventional TRA puncture, also, thus necessitating crossover, regardless22. These included the presence of severe proximal radial loops, vessel tortuosity, and aberrant/tortuous subclavian vessels. The overall high success rate in the present audit could be explained, in part, by operator experience – a sizable caseload was conducted by attending/consultant interventional cardiologists rather than cardiology fellows/registrars. This notion is supported by our logistic regression model as well as in the literature; both Li et al23 and a recent RCT by Lee et al16 cite operator proficiency as an independent determinant of procedural success. As dTRA puncture is still relatively novel, it could be projected that its success rate will improve with time and a more widespread adoption by the interventional community.
It is worth mentioning that PCI chiefly involving the left main coronary artery, bifurcation disease, and heavily calcified lesions typically requires larger-bore catheter sizes. There is some debate in the literature as to whether the smaller-diameter dTRA can adequately facilitate these procedures as smoothly as the TRA2425. The median sheath size we employed was 6 Fr, with over one-third of all-comers undergoing successful PCI, including for emergency indications such as ST-segment elevation myocardial infarction and cardiac arrest. PCI with a larger-bore access (≥7 Fr) was utilised in 212 cases (36% of PCI), only 10 (4.7%) of which required access crossover to a larger-diameter vessel. Furthermore, the need for a greater sheath size to aid PCI of a large coronary artery, specifically, was quoted as a reason for puncture failure in only 3 cases. This is likely due to increasing access to thin-walled sheaths and sheathless systems. Subsequently, the rate of radial artery-to-sheath size mismatch is reduced, which facilitates successful PCI via the dTRA, even with relatively large-diameter catheters26. Therefore, the data suggest that the dTRA permits coronary angiography and PCI despite its smaller dimension, with an acceptable rate of access failure and crossover.
Safety
The safety profile of dTRA puncture as conveyed in our study is further supported by RCTs and meta-analyses comparing dTRA versus conventional TRA approaches212728. No patients in the study experienced major vascular complications, including major bleeding, vascular surgery or digital/hand ischaemia. Furthermore, the need for bailout femoral access was minimal, at approximately 1 in 300 patients. Notably, few patients experienced distal RAO, and of these, only two experienced proximal RAO, which highlights an important patient benefit for dTRA access. Studies have shown that dTRA puncture may also be used as a means to recanalise a proximal radial occlusion without significantly increasing the procedure time, volume of contrast used, or rate of PCI success293031, highlighting its clinical utility. Therefore, dTRA access during the index procedure not only demonstrates a clear benefit in attenuating vascular complications, but it appears favourable in those who may later require repeat arterial catheterisation, such as younger patients, those with diabetes or higher risk of target vessel failure, or potentially even those with forearm proximal RAO after a previous procedure.
Predictors of puncture success
The diameter of the dTRA has been shown to vary according to age, sex and ethnicity3233. Our regression model highlighted hypertension, a low BMI, and operator experience as predictors of puncture success. A limitation of our study is that data on radial artery diameter, per ultrasound, and data pertaining to ethnicity were unavailable. It is possible that BMI, hypertension, and sex are confounders as they correlate with the radial artery diameter. We hypothesise that the mechanism of hypertension upon puncture success was twofold: via the increased diameter of the elastic peripheral arteries related to adaptive remodelling in hypertension and, secondly, by causing a stronger pulse34. Firstly, vessel calibre is an independent predictor of success in multiple studies11333536, with one study attributing this to hypertension35. Conversely, hypertension was shown to negatively correlate with dTRA puncture success in another study36. In the latter, the authors hypothesised this to be secondary to a high concomitant atherosclerotic burden that narrowed the intimal layer; however, this was likely to be confounded because of age. The second mechanism is supported in the recent KODRA trial, which reports a weak pulse as a predictor of dTRA puncture failure16. Ultrasound guidance has been shown to increase dTRA access success, and thus, its routine use in angiography has been advocated33. The dTRA is best located by running a linear transducer probe perpendicularly to the skin, from the first dorsal webspace to the anatomical snuffbox. Once visualised, colour Doppler should be applied to ensure vessel patency37. We hypothesised that high BMIs were correlated with a deeper dTRA, poorer arterial visualisation, and a subsequent more challenging puncture, compared to low BMIs. In contrast, there is literature that reports a high BMI as a facilitator of successful puncture, given its positive association with the dTRA diameter1138; furthermore, the converse has also been recognised: a low BMI leads to failure394041. The strongest predictor of success was operator experience, as previously discussed16.
Limitations
This was a retrospective cohort study, and selection bias is unavoidable. For example, taller patients (>185 cm) are preferentially catheterised using the conventional approach in our centres. The snuffbox is approximately 5 cm below the common radial entry site42, and therefore, a radial catheter introduced via the dTRA may not reach the coronary ostia. A limitation of our study is that the high first-pass success rate (92.2%) is based on an operator-reported metric, which is prone to bias, and our definition includes minor needle adjustments and multiple wire passage attempts within the initial puncture, further complicating the comparability of our results with other studies using different definitions. Furthermore, follow-up data were not captured for a large proportion of the cohort, meaning that the true rate of RAO and suitability for repeat puncture may be underestimated.
Conclusions
Coronary angiography and PCI via the dTRA appear to be safe and feasible when performed by highly experienced and skilled operators. Hypertension, operator experience and a low BMI were predictors of procedural success.
Impact on daily practice
The distal transradial artery is a safe and effective first-line vascular access site. To increase the rate of successful first pass access and reduce the risk of subsequent complications from failed and/or traumatic punctures, patient comorbidities should be considered when selecting the most appropriate vascular access approach. Patients with diagnosed hypertension and low body mass index are independent predictors of first-pass distal transradial approach success. Operator experience is also associated with increased likelihood of procedural success; the overall success rate of this technique will improve with time and a more widespread adoption by the interventional community.
Acknowledgements
The authors would like to express their gratitude to all the patients and cardiac catheterisation staff who assisted with the study.
Conflict of interest statement
The authors have no conflicts of interest to declare.
